19. Transplant hepatology: a comprehensive update
Richard Taubert, Theresa Kirchner
(based on the previous issue by Susanne Beckebaum et al.)
Abstract
Liver transplantation (LT) is the only life-saving therapy in patients with advanced liver disease, cirrhosis or acute liver failure. Although LT is a true success story, a multiprofessional team in a specialised centre is needed for patient selection, waiting list monitoring and surveillance after LT. In nowadays new techniques expand the pool of organs in times of organ shortage. Individualised immunosuppression regimes should be used to improve graft and patient survival and to reduce side effects due to immunosuppressive medication. Treatment of recurrence of underlying disease could be challenging.
Hereinafter we will give an overview over indications for LT, pre- and posttransplant patient management, risk factors before and after LT and treatment of complications.
Introduction
Over the past 30 years major advances have been made in the field of organ transplantation due to improvements in surgical techniques and organ conservation as well as optimisation of intensive care and immunosuppressive management. This chapter focuses on important issues in the field of transplant hepatology and may provide helpful information to physicians involved in the care of adult liver transplant (LT) recipients. It includes indications for LT, current organ allocation policy, pretransplant evaluation, management while on the waiting list, living donor liver transplantation (LDLT) and management of early and long-term complications post-LT.
Timing and indications for liver transplantation
Appropriate selection of candidates and timing of LT is crucial in reducing mortality and improving outcomes in LT recipients. A patient is considered too healthy to undergo LT if the expected survival is longer without surgery. Therefore, criteria are needed in order to select patients with priority for LT who can most benefit from transplantation. In 2002, the Organ Procurement and Transplantation Network along with the United Network of Organ Sharing (UNOS) developed a system based on the model for end-stage liver disease (MELD) (Table 1) to prioritise patients on the waiting list. In the Eurotransplant countries, the Child-Pugh Turcotte (CPT) score was replaced by the MELD score in December 2006.
The lab MELD score using the three laboratory parameters depicted in Table 1 ranges from 6 (less ill) to 40 (severely ill). It estimates mortality in patients with end stage liver disease within 90 days (Kwong 2015). The MELD score is used for candidates 12 years of age or older and the Paediatric End Stage Liver Disease Model (PELD) score is used for patients <12 years of age. The MELD score includes creatinine, total bilirubin and INR, age is added to PELD. In a large study (Merion 2005) looking at the survival benefit of LT candidates, those transplanted with a MELD score <15 had a significantly higher mortality risk as compared to those remaining on the waiting list, while candidates with a MELD score of 18 or higher had a significant transplant benefit.
Table 1. Calculation of the MELD* Score| MELD Score = | 10x (0, 957 x ln [creatinine mg/dL] + 0, 378 x ln [total bilirubine mg/dL] + 1, 12 x ln [INR**] + 0, 643) |
The MELD score does not accurately predict mortality in approximately 15-20% of patients. Therefore MELD-based allocation allows exceptions for patients whose score may not reflect the severity of their liver disease. These exceptions include e.g. hepatocellular carcinoma (HCC), nonmetastatic hepatoblastoma, adult polycystic liver degeneration, primary hyperoxaluria type 1, small-for-size syndrome, cystic fibrosis, familial amyloid polyneuropathy, hepatopulmonary syndrome, portopulmonary hypertension, urea cycle disorders, hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease), hemangioendothelioma of the liver, biliary sepsis, primary sclerosing cholangitis (PSC) and cholangiocarcinoma. Patients with standard exceptions will be assigned a higher MELD score (match MELD) than that assigned by the patient’s laboratory test results (lab MELD). Consequently, this resulted in an increasing proportion of patients transplanted for HCC and other exceptions over time (Massie 2011).
MELD has proved to be accurate as a predictor of waiting list mortality, but has shown to be less accurate in predicting posttransplant outcome (Kaltenborn 2015). For instance, MELD allocation resulted in decreased waiting list mortality; whereas posttransplant morbidity has increased due to transplantation of a higher proportion of sicker recipients with MELD scores >30 (Dutkowski 2011). Moreover, the quality of donor organs has been impaired over the last two decades (Schlitt 2011).
Creatinine values exert a systematic bias against women due to their lower creatinine values conditioning a longer waiting time for an organ (Rodríguez-Castro 2014). Thus women are disadvantaged by use of MELD score in terms of access to LT. The question has been raised whether additional candidate characteristics should be explicitly incorporated into the prioritisation of waiting list candidates (Sharma 2012). It has also been suggested to take into account not only pretransplant mortality but also donor-related factors for estimation of the donor risk index (DRI) (Feng 2006) and posttransplant mortality. Furthermore, standardisation of laboratory assays and variants of MELD including incorporation of parameters such as sodium or cholinesterase have been proposed to overcome the limitations of the current scoring system (Choi 2009, Weissmüller 2008, Vitale 2012). The Hong Kong transplant group aimed to establish additional criteria to predict short-term mortality in severe flares of chronic hepatitis B virus (HBV) infection (Fung 2019). Their results revealed that HBV-infected patients with MELD ≥28 should be worked up for LT, and those with MELD 28-32 with 3-4 at-risk criteria (age ≥52 years, ALT >217 U/L, platelets <127, and abnormal baseline imaging), or MELD ≥32 should be listed.
UNOS made a policy change and revised the MELD scoring system on January 11, 2016 by incorporating the serum sodium value (MELD-Na) because patients with hyponatraemia have significantly higher mortality rates compared with those with normal serum sodium levels. But the MELD-Na also appears to disadvantage women in the waiting list. Because of this Wood et al. designed a corrected MELD-Na that eliminates sex disparities (Wood 2021).
Candidates for LT must have irreversible acute or chronic end-stage liver disease. Alcohol-induced liver disease (ALD, 35.2%) and viral infections (34.9%) have been the most common disease indications in adults with liver cirrhosis (https://www.eltr.org) during the last decades (Figure 1). Non-alcoholic fatty liver disease (NAFLD) is a frequent aetiology of liver disease in western countries and has become a leading indication for LT in the United States (US) and Europe; whereas the proportion of transplant waitlist additions for HCV-associated disease has declined since the introduction of interferon-free, direct-acting antiviral (DAA) therapy (Cotter 2019). Data from the UNOS and Organ Procurement and Transplantation Network registry from 2004 through 2013 revealed that the number of adults with non-alcoholic steatohepatitis (NASH) awaiting LT has almost tripled since 2004 (Wong 2015).
Other indications include cholestatic liver disorders (primary biliary cirrhosis [PBC], PSC), HBV infection, autoimmune hepatitis (AIH), inherited metabolic diseases (Wilson’s Disease, haemochromatosis, α-1-antitrypsin deficiency), HCC, and acute or acute-on-chronic hepatic failure. In children, biliary atresia and metabolic liver diseases are the most common indications. Contraindications for LT include extrahepatic malignancies, sepsis, uncontrolled pulmonary hypertension, and coexistent medical disorders such as severe cardiopulmonary condition, technical or anatomical barriers such as thrombosis of the entire portal and superior mesenteric venous system. Previous malignancy history must be carefully considered and likelihood of recurrence estimated. Active alcohol consumption is a relative contraindication, because more and more studies show the life saving effect with acceptable alcohol relapse rates after liver transplantation in severe and refractory manifestations of alcoholic hepatitis in highly selected patients (Mathurin 2011, Lee (c) 2018, Carrique 2021).
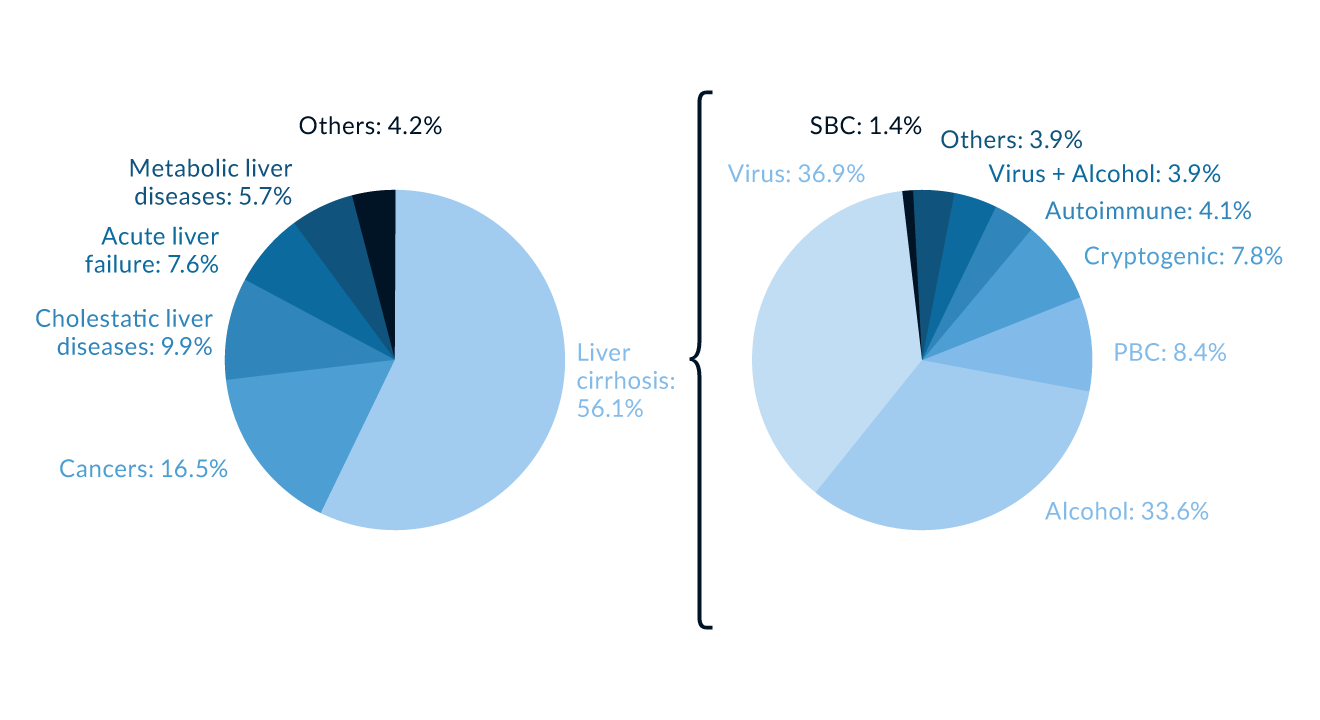 Figure 1. Indications for liver transplantation (LT). Primary diseases leading to LT in Europe, 1988–2015 (Data kindly provided from European Liver Transplant Registry, https://www.eltr.org)
Figure 1. Indications for liver transplantation (LT). Primary diseases leading to LT in Europe, 1988–2015 (Data kindly provided from European Liver Transplant Registry, https://www.eltr.org)PBC = primary biliary cholangitis SBC = secondary biliary cirrhosis
Patient evaluation
Evaluation of a potential transplant candidate is a complex and time-consuming process that requires a multidisciplinary approach. Requirements for evaluation may differ slightly between transplant centres. The evaluation process must identify extrahepatic diseases that may exclude the patient from transplantation or require treatment before surgical intervention. The protocol we use for evaluation of potential transplant candidates is shown in Table 2.
Pretransplant management issues
In cases of recurrent variceal hemorrhage despite prior interventional endoscopic therapy (and non-selective beta-blockade) or refractory ascites, transjugular intrahepatic portosystemic shunts (TIPS) have been used to lower portal pressure and as bridging therapy for transplant candidates. The identification of predisposing factors and medication such as lactulose and rifaximin, a minimally absorbed antibiotic, are effective for prophylaxis and management of hepatic encephalopathy (HE) (Mullen 2014).
Hepatorenal syndrome (HRS) represents a complication of end-stage liver disease and is a risk factor for acute kidney injury (AKI) in the early post-operative phase (Saner 2012). It is classified into type 1 HRS characterised by a rapid impairment of renal function with a poor prognosis; type 2 HRS is a moderate steady renal impairment. Vasoconstrictors including terlipressin in combination with volume expansion are commonly used and have been shown to be effective for restoration of arterial blood flow and serve as bridging therapy to LT (Hinz 2013). Extracorporeal liver support systems based on exchange or detoxification of albumin have been successfully employed in indicated cases.
Beyond MELD, other parameters such as frailty and sarcopenia might be essential to consider suitable patients for the waiting list. Sarcopenia is part of the frailty complex present in cirrhotic patients. According to the operational definition by the European Working Group on Sarcopenia in Older People (EWGSOP), the diagnosis of sarcopenia comprises the presence of both low muscle mass and low muscle function in terms of low muscle strength or low physical performance. Muscle wasting is considered one of the major complications of end-stage liver cirrhosis and may be caused by a variety of factors such as reduced nutrient intake, dietary restrictions in sodium and water in decompensated liver disease, reduced protein intake for hepatic encephalopathy, reduced intestinal absorption secondary to maldigestion caused by pancreatic exocrine insufficiency or to intestinal bacterial overgrowth due to small bowel motility disorders and a hypermetabolic state with increased energy consumption and high protein catabolism.
Sarcopenia was highly associated with waitlist mortality and negative perioperative outcome (Kahn 2018, Meeks 2017). This was in particular an issue in patients who were listed with low priority based on a low MELD score (van Vugt 2017).
After waitlisting, laboratory values must be updated according to the recertification schedule shown in Table 3.
Table 2. Basic (not exhausted) evaluation protocol for potential transplant candidates| Physical examination |
| Diagnostic tests (baseline laboratory testing; serologic, tumour/virologic, and microbiological screening; coagulation tests, autoantibodies; thyroid function tests) |
| Abdominal ultrasound with vascular Doppler/Duplex |
| Abdominal MRI or computer tomography (CT) scan |
| Chest X-rays |
| Electrocardiogram (ECG), cardio CT in patients ≥50 years or > 2 cardiological risk factors, cononary angiography only if indicated and after cardio CT, Swan-Ganz catheterisation, Doppler/Duplex carotid arteries |
| Upper and lower endoscopy |
| Pulmonary function testing |
| Mammography (in females >50 years) |
| Physician consultations (anesthesiologist, gynecologist, urologist, cardiologist, neurologist, dentist, ear, nose, and throat specialist) |
| A meticulous psychosocial case review (medical specialist in psychosomatic medicine, psychiatry or psychology) |
| Score | Recertification | Lab values |
| ≥25 | every 7 days | ≤48 hours old |
| 24–19 | every 30 days | ≤7 days old |
| 18–11 | every 90 days | ≤14 days old |
| ≤10 | every year | ≤30 days old |
Special attention regarding specific, disease-related therapy prior to surgery should be given to transplant candidates undergoing LT for HCC or virally-related liver diseases.
Waiting list monitoring of patients with ALD
ALD is currently the most common indication for LT in many European and US LT centres. The 6-month abstinence requirement (the so-called '6-month rule') is a common practise requiring candidates abstinent from alcohol for at least 6 months to be eligible for transplant.
ALD is associated with a lower risk of waitlist removal for deterioration (HR 0.84, 95%CI 0.81-0.86, p<0.001) and a higher risk of waitlist removal for improvement (HR 2.91, 95%CI 2.35-3.61 p<0.001) as compared to non-ALD (Giard 2019).
Alcoholic hepatitis (AH) represents a subpopulation of patients with ALD with short term mortality approaching 70% in severe cases. The thresholds for amount and duration of alcohol use leading to severe AH (SAH) are not clearly defined. However, an average consumption of more than 40 g per day for women and 50–60 g per day for men are estimated minimum thresholds for the diagnosis of SAH. Heavy alcohol use has usually occurred for >6 months (typically for several years) with <2 months of abstinence before clinical presentation of jaundice.
Until recently, LT as a treatment for SAH has been a taboo in most transplant centres owing to concerns about the limited organ supply and the risk that the SAH liver recipient will return to harmful drinking. Moreover, there has been a controversial discussion in literature about LT in SAH (Fung 2017, Lucey 2017, Barosa 2017, Daswani 2018, Kubiliun 2018, Lee (a) 2018, Zhu 2018, Mitchell 2019, Thursz 2018), and this issue has been debated in national and international conferences and liver societies (Addolorato 2016, Martin 2014, EASL CPG 2018: management of alcohol-related liver disease, Graziadei 2016).
The change in attitude has been launched by a French-Belgian study group (Mathurin 2011) which favoured early LT in SAH as a reasonable rescue option for patients who failed to respond to conservative therapy. The authors selected patients who had no prior episodes of AH and had scores ≥0.45 according to the Lille model or rapid deterioration of liver function despite medical therapy. Only patients were selected who had family support, no severe comorbidities and were commited to alcohol abstinence. Only 2.9% of available grafts were considered for this indication. The cumulative 6-month survival rate (±SE) was significantly higher among patients undergoing early LT than among those who were not placed on the waiting list (77 ± 8% vs. 23 ± 8%, P<0.001). This was also true through 2 years of follow-up (hazard ratio, 6.08; P = 0.004). Three patients had an alcohol relapse at 720 days, 740 days, and 1140 days after LT.
A lively international debate about the selection criteria in patients with ALD was sparked in 2012. An advantage of the 6-month period of abstinence before listing is avoidance of unnecessary LT in patients who will spontaneously improve and a commitment of the patient to abstinence giving the opportunity to implement preventive strategies against future relapse episodes (Im 2019). Arguments in favour for LT is the risk of death in patients with severe ALD/AH, the fact that the 6-month rule as a single predictor of abstinence is debatable and may discriminate patients with favourable prognosis and low risk of recurrence. A multicentre control study from French and Belgian with 149 patients cannot conclude non-inferiority in terms of rate of alcohol relapse post-transplant between early liver transplantation and standard transplantation (after at least six month of abstinence). The prospective controlled study confirms the important survival benefit in early liver transplantation in patients with severe alcohol-related hepatitis but high alcohol intake is more frequent after early liver transplantation (Louvet 2022).
The majority of LT recipients after LT for AH maintains long-term abstinence, but younger age, multiple prior rehabilitation attempts and overt encephalopathy were associated with post-LT alcohol use (Lee (d) 2022). Further suggested predictors of recurrence include positive family history of substance use, alcohol-related comorbidity, history of prior alcohol-related legal issues, history of substance abuse (other than alcohol), lack of social support, lack of familiar support, denial of drug-related problems and addiction length and intensity of ALD. Prognostic instruments used to predict future drinking after LT include the University of Michigan Alcoholism Prognosis score, the Alcohol Relapse Risk score, the High Risk Alcoholism Relapse (HRAR) score and the Stanford Integrated Psychosocial Assessment for Transplantation (SIPAT) (Im 2019). However these scores were not specifically developed for the LT setting. Therefore, Lee et al. (b) (2019) developed a new prognostic score (SALT score) using 4 pretransplant variables to identify AH candidates at low risk for alcohol relapse after early LT. A multidisciplinary approach including psychosocial and medical assessment and integration of an addiction specialist may be a crucial prerequisite to properly determine suitability of the ALD patient for LT. In nowadays even artificial intelligence is used to identify harmful alcohol use after LT by psychological profiles (Lee (e) 2022).
Results of several studies and retrospective analyses resulted in a paradigm shift in therapy for highly selected patients with SAH who are not responding to medical therapy. The UNOS, the EASL Clinical Practise Guideline on alcohol-related liver disease (2018) and the American College of Gastroenterology (ACG) Clinical Guideline (Singal 2018) therefore suggest that the decision for waitlisting should not be based only on the 6-month abstinence rule. Presently, in case of non-response to conservative therapy, highly selected patients can therefore be considered for early LT in European and US transplant centres (Antonini 2018, Lee (c) 2018, Thurs 2019, Carrique 2021).
Addiction rehabilitation programmes should be implemented prior to LT, and post-LT contracting, for alcohol after care and counseling should be considered in patients who are too sick to attend pretransplant rehabilitation treatment.
Management of patients with ALD in the context of LT is an ongoing debate in Germany. According to legally binding guidelines of the German Medical Association abstinence must be proven by negative urine ethyl glucuronide (uETG) tests (and hair-ETG/carbohydrate-deficient Transferrin (CDT) if applicable) during the 6 months before possible waitlisting (https://www.bundesaerztekammer.de/fileadmin/user_upload/BAEK/Ueber_uns/Richtlinien_Leitlinien_Empfehlungen/RiliOrgaWlOvLeberTx20230121.pdf). Furthermore, a positive psychiatric assessment with potential recommendations for psychotherapeutic measures is mandatory before listing. As soon as a patient is on the waiting list due to ALD, ETG testing is required at every visit in the LT outpatient clinic (at least every 3 months).
The majority of patients with severe SAH already reveal cirrhotic changes of the liver in terms of acute on chronic liver failure and do not meet the 6-months rule. In exceptional urgent cases the transplant conference of the corresponding German LT centre can deviate from the 6-months rule (https://www.bundesaerztekammer.de/fileadmin/user_upload/BAEK/Ueber_uns/Richtlinien_Leitlinien_Empfehlungen/RiliOrgaWlOvLeberTx20230121.pdf). This presupposes a request by the transplant centre for an alcohol audit which is carried out by a committee of specialists nominated by the German Medical Association. Eurotransplant organises the audit process consisting of 3 auditors who give an expert opinion (independently of each other). A positive vote is achieved if all 3 auditors agree to an exceptional listing. However, after completion of the audit process the transplant conference takes the final decision to list or not to list the patient
Psychosocial interventions should be routinely used in the medical management of ALD prior to and after LT (EASL CPG: Liver transplantation [2016]). Once listed, patients with ALD should be monitored for alcohol use by clinical interviewing and random biochemical testing. The specific biochemical test used in different countries and transplant centres will depend on availability, programme resources and costs. Currently, anticraving drugs (except baclofen) and disulfiram are not recommended in patients with advanced ALD, because of the potential side effects and insufficient experience in this population.
Waiting list monitoring and treatment of viral hepatitis B and C in liver transplant candidates
The treatment of viral hepatitis B and C is well established and patients should be treated according to actual guidelines. In all viremic patients with viral hepatitis B on the waiting list efficient therapy should be started. The goal of antiviral therapy in HBV patients on the waiting list is to achieve viral suppression to undetectable HBV DNA levels using sensitive tests (Cornberg 2011, Beckebaum 2013a). Several studies have demonstrated clinical benefits in patients with decompensated cirrhosis with viral suppression as reflected by a decrease in CPT score, improvement of liver values and resolution of clinical complications (Kapoor 2000, Schiff 2007). Moreover, initiation of nucleos(t)ide analogue (NUC) treatment prior to LT has markedly reduced HBV recurrence posttransplantation.
The success of direct-acting antivirals (DAAs) has dramatically changed the landscape for HCV and liver transplantation. The diagnosis of a decompensated liver cirrhosis with replicative hepatitis C is rarity nowadays. Only very few patients have to be transplanted with a replicative hepatitis C and need a DAA therapy after liver transplantation. Nearly all liver transplant patients with a reinfection of HCV in the past reached a sustained virological response with DAA therapy.
According to theEuropean Association for the Study of the Liver (EASL) Recommendations on Treatment of Hepatitis C (2020), patients without cirrhosis and with compensated (Child-Pugh A) cirrhosis without HCC awaiting LT with a MELD score <18-20 should be treated prior to LT; whereas those without HCC and a MELD score ≥18-20 should be transplanted first without antiviral treatment. Patients with decompensated cirrhosis (Child-Pugh B or C) without HCC awaiting LT with a MELD score <18–20 have an indication for antiviral treatment with the fixed-dose combination of sofosbuvir, velpatasvir and daily ribavirin. In HCV transplant candidates with HCC timing of antiviral therapy should not interfere with the management on the waiting list, it must be decided on a case-by-case basis. Patients with HCC without cirrhosis or with compensated cirrhosis should be treated for HCV infection prior to LT.
Based on available data and according to EASL recommendations (2020) the use of HCV-infected organs is acceptable in patients at high risk of death on the waiting list but should not be offered to non-infected recipients with a MELD score <20 if there is no access to anti-HCV therapy.HCV negative patients receiving a HCV positive organ should be treated in any case.
Adjunct treatment and staging of HCC transplant candidates
LT should be considered in early or intermediate stage HCC (Reig (b) 2022). A 5-year survival rate of 75–80% can be achieved in patients with HCC undergoing LT (Vogel (b) 2022). Under MELD allocation, patients must meet the Milan criteria (one tumour ≤5 cm in diameter or up to three tumours, all ≤3 cm, no extrahepatic manifestation, no macrovascular infiltration) to qualify for exceptional HCC waiting list consideration. Diagnosis of HCC is confirmed if the following criteria are met according to the German Medical Association (https://www.bundesaerztekammer.de/fileadmin/user_upload/BAEK/Ueber_uns/Richtlinien_Leitlinien_Empfehlungen/RiliOrgaWlOvLeberTx20230121.pdf): (1) liver biopsy-proven alone or (2) two contrast-enhanced (CE) imaging techniques (CE-magnetic resonance imaging [MRI], CE- computed tomography [CT] or CE-ultrasound [US]) in tumours 1 cm up to ≤2 cm; (3) one contrast-enhanced imaging technique (CE-MRI, CE-CT) in tumours >2 cm; (4) arterial hypervascularisation with rapid venous wash out, displaying contrast reversal in comparison to the surrounding liver tissue in 3-phase cross-sectional imaging techniques. Initial imaging (before downstaging with interventional therapy or resection) has to be used for diagnosis. Patients are registered at a MELD score equivalent to a 15% probability of pretransplant death within 3 months. Patients will receive additional MELD points equivalent to a 10% increase in pretransplant mortality to be assigned every 3 months until these patients receive a transplant or become unsuitable for LT due to progression of their HCC. The listing centre must enter an updated MELD score exception application in order to receive additional MELD points.
Pre-listing, the patient should undergo a thorough assessment to rule out extrahepatic spread and/or vascular invasion. The assessment should include CT scan or MRI of the abdomen, pelvis and chest. We perform trimonthly routine follow-up examinations (MRI or CT scan) of waitlisted HCC patients for early detection of disease progression. Underestimation of HCC burden before LT has shown to be frequent despite advanced imaging technologies. This has been reconfirmed in a study conducted by Ecker et al. (2018). The authors collected HCC patients who underwent LT after preoperative MRI in a prospective institutional database (January 2003 to December 2013). Patients were subdivided in those “within” or “outside” Milan criteria by both imaging and explant pathologic evaluation. Of 318 patients with HCC meeting Milan criteria by MRI at the time of LT, only 248 (78.0%) remained within Milan on explant examination.
Waiting list drop-out rates can be reduced by the application of bridging therapies such as transarterial chemoembolisation (TACE) or radiofrequency ablation (Roayie 2007, Reig (b) 2022). In patients treated with transarterial chemoembolisation before LT for HCC Response Evaluation Criteria in Solid Tumours (RECIST) have shown to be superior to EASL criteria at 1 month follow-up for predicting long-term survival (Shuster 2013). Transarterial radionuclide therapies such as Yttrium-90 microsphere transarterial radioembolisation (TARE) have been tested for bridging therapy in selected cases (Toso 2010).
Kulik et al. (2018) aimed to investigate the effectiveness of locoregional therapy (LRT) in LT candidates with HCC on the LT waitlist. They conducted a systematic review and metaanalysis considering multiple databases from 1996 to April 25, 2016, for studies that enrolled adults with cirrhosis awaiting LT and treated with bridging or down-staging therapies before LT. LRT included TACE, transarterial radioembolisation, ablation, and radiotherapy. The authors showed that in LT candidates with HCC, the use of LRT is associated with a nonsignificant trend toward improved waitlist and posttransplant outcomes. Bridging therapy should be considered in particular in patients outside the Milan criteria, with a likely waiting time of longer than 6 months, and those within the Milan criteria with high-risk characteristics of HCC. Sorafenib has been administered in a few studies before LT to investigate the safety and efficacy of this oral multikinase inhibitor in the neoadjuvant setting (Fijiki 2011, Di Benedetto 2011). A systematic review of the few available studies showed that perioperative use of sorafenib did not improve patient survival and could even lead to a worse prognosis (Qi 2015). Moreover, sorafenib is frequently associated with side effects such as fatigue, weight loss, skin rash/desquamation, hand–foot skin reaction, alopecia and diarrhoea, requiring dose reduction or treatment discontinuation. Accurate discrimination of HCC patients with good and poor prognosis by specific criteria (genomic or molecular strategies) is highly warranted to select appropriate treatment options (Bittermann 2014, Tournoux-Facon 2011).
Lately immune check point inhibitors were established in the individualised HCC treatment as standard of care (Vogel (b) 2022). The combination of atezolizumab with bevacizumab is currently the firt choice first-line treatment, liver function has to be preserved and bleeding risk should be low in this patient group (Reig 2022). There is still an ongoing discussion if check point inhibitors should be used before transplantation and when.
Liver transplantation in autoimmune hepatitis and cholestatic liver diseases
In Europe 4% of cirrhosis patients were transplanted due to AIH and 8% due to PBC, based on the data from the European Liver Transplant Registry (https://www.ELTR.org).
An international multicentre study of 3, 902 PBC patients, Harms et al (2019) found that treatment with UDCA is associated with prolonged liver transplant-free survival.
On the one hand AIH could lead to chronic liver failure due to cirrhotic liver impairment but on the other hand acute severe autoimmune hepatitis can lead to acute liver failure. The management and the right timing for LT in patients with severe acute AIH is still challenging. In a retrospective multicentre study by De Martin et al (De Martin 2021) acute severe AIH was diagnosed by definite or probable AIH based on the simplified AIH score, an INR ≥ 1.5 and/or bilirubin >200 μmol/L, no previous history of AIH and a histologically proven AIH. The study showed that in patients with acute severe AIH the INR at the introduction of corticosteroids and the evolution of INR and bilirubin are predictive of LT or death. A new scoring system (SURFASA score) was built. The score comprised three parameters: INR at baseline, change in INR over 3 days and change in total bilirubin over 3 days after beginning of steroid treatment, the cut off point was <-0.9. Responding rate on steroid therapy was 75% below this cut off and with a score >1.75 the risk of dying or LT was 85-100%. The score was validated later, but the authors highlight that traditional MELD score were equally accurate (Lin 2022).
PSC, accounting for approximately 5% of all transplant cases, is a rather small indication group on the waiting list. According to the actual Guidelines of the German Medical Association, patients with PSC who fulfil the standard exception criteria receive a match MELD reflecting the sum of 3-month mortality according to lab MELD and a 15% 3-month mortality at listing and then they are upgraded every three months following every 10% increase of the 3-month mortality (https://www.bundesaerztekammer.de/fileadmin/user_upload/BAEK/Ueber_uns/Richtlinien_Leitlinien_Empfehlungen/RiliOrgaWlOvLeberTx20230121.pdf). A large retrospective study with 286 PSC patients by Rupp et al showed that the rate of transplantation-free survival was higher in patients receiving scheduled ERCP compared to patients with ERCP on demand (Rupp 2019). However benefit was only significant in patients with the initial or later diagnosis of a dominant stenosis, even if asymptomatic. Another large multicentre study (2975 PSC patients from 27 centres) highlights that scheduled imaging (ultrasound and/or MRI) improves survival in PSC (Bergquist 2023). Asymptomatic patients with cholangiocellular carcinoma hat a better survival if scheduled imaging had been performed (Bergquist 2023).
Living donor liver transplantation: indications, donor evaluation, and outcome
LDLT was introduced in 1989 in a successful series of paediatric patients (Broelsch 1991). Adult-to-adult LDLT (ALDLT) was first performed in Asia where cadaveric organ donation is rarely practiced (Sugawara 1999, Kawasaki 1998). LDLT peaked in the US in 2001 (Qiu 2005) but thereafter the numbers declined by 30% over the following years (Vagefi 2012, Carlisle 2012). A decline over time was also observed in Europe, whereas LDLT activity increased in Asia. Recently published studies showed good survival rates in HCC-patients with LDLT beyond Milan compared to those within Milan (Alim 2021, Liang 2021). In the last years LDLT is increasingly mentioned in various indications.
In selected cases, LDLT offers significant advantages over deceased donor LT (Quintini 2013). The evaluation of donors is a cost-effective and time-consuming process. Clinical examinations, imaging studies, special examinations, biochemical parameters, and psychosocial evaluation prior to donation varies from centre to centre and has been described elsewhere (Valentin-Gamazo 2004). Using Germany as an example, the expenses for evaluation, hospital admission, surgical procedure, and follow-up examinations of donors are paid by the recipient’s insurance. Due to the increasing number of potential candidates and more stringent selection criteria, rejection of potential donors has been reported in 69-86% of cases (Valentin-Gamazo 2004, Pascher 2002). The advantages of LDLT include the feasibility of performing the operation when medically indicated and the short duration of cold ischaemia time.
LDLT is associated with surgical risks for the recipient AND donor (Baker 2017). The surgical procedures for LDLT are more technically challenging than those for deceased donor LT. In the recipient operation, bile duct reconstruction has proven to be the most challenging part of the procedure with biliary complications ranging from 15% to 60% (Sugawara 2005).
Regarding donor outcome, morbidity rates vary considerably in the literature (Patel 2007, Beavers 2002, Shiraz 2016). Possible complications include wound infection, pulmonary problems, vascular thrombosis with biliary leaks, strictures, and incisional hernia. A major concern related to LDLT is still donor safety because an operative procedure with potential risks must be carried out on a healthy individual (Baker 2016). Biliary complications are the most common postoperative complication in LDLT and occur in up to 7% of donors (Perkins 2008, Sugawara 2005). Liver regeneration can be documented with imaging studies and confirmed by normalisation of bilirubin, liver enzymes, and synthesis parameters. Morbidity rates are strongly related to the experience of the surgical team and should be performed only by established transplant centres with appropriate medical expertise. The currently reported postoperative mortality rates for left and right hepatectomy are 0.1% and 0.5 %, respectively. Outcome in patients undergoing LDLT is similar if not even better than in those undergoing deceased donor LT (Nadalin 2015, Alim 2021).
Perioperative complications
Cardiac decompensation, respiratory failure following reperfusion, and kidney failure in the perioperative LT setting constitute major challenges for the intensive care unit. Acute kidney injury (AKI) has a major impact on short- and long-term survival in LT patients. For instance, Pulitano et al. (2018) found that AKI was associated with increased risk of early allograft dysfunction and chronic kidney disease stage ≥ 2 posttransplant.
There is no currently accepted uniform definition of AKI, which would facilitate the standardisation of care of patients with AKI and improve and enhance collaborative research efforts. Biomarkers such as neutrophil gelatinase-associated lipocalin or kidney injury molecule-1 have been developed for the prevention of delayed AKI treatment (Saner 2012). Moreover, genetic profiling of post-reperfusion milieu showed that endothelin-1 and interleukin-18 serum levels on postoperative day 1 were independent predictors of AKI in multivariate analysis (Pulitano 2018).
Early dialysis has been shown to be beneficial in patients with severe AKI (stage III according to the classification of the Acute Kidney Injury Network) (Bellomo 2004), whereas treatment with dopamine or loop diuretics have shown to be associated with worse outcome. Preventative strategies of AKI include avoidance of volume depletion and maintenance of a mean arterial pressure >65 mm Hg (Saner 2012).
Despite advances in organ preservation and technical procedures, postoperative complications due to preservation/reperfusion injury have not markedly decreased over the past several years. Typical histological features of preservation and reperfusion injury include centrilobular pallor and ballooning degeneration of hepatocytes. Bile duct cells are more sensitive to reperfusion injury than hepatocytes (Washington 2005) resulting in increased serum levels of bilirubin, gamma-glutamyl transpeptidase (GGT) and alkaline phosphatase (AP). A recently published randomised trial showed that hypothermic Machine Perfusion in LT leads to a lower risk of non-anastomotic biliary strictures after LT and reduces the rate of postreperfusion syndrome and early allograft dysfunction (van Rijn 2021). Machine perfusion expands the pool of usable livers dramatically and improves graft function (Sousa Da Silva 2022; Czigany 2021, Brüggenwirth 2022).
Vascular complications continue to have devastating effects. In deceased LT, overall vascular complications such as hepatic artery thrombosis (HAT) have been reported in 1.6-4% of patients. Shiraz et al. (2016) retrospectively analysed the trends observed in vascular complications with changing protocols in adult LDLT (A-LDLT) and paediatric LDLT (P-LDLT) over 10 years. Depending on the era of LT the authors stratified the cohort in Group I (n= 391, Jan. 2006- Dec.2010) and Group II (n=741, Jan. 2011- Oct. 2013) patients. With a minimum follow up of 2 years, incidence of HAT in adults has reduced significantly from 2.2% in Group I to 0.5% to Group II, p = 0.02. In Group II non-significantly more adult patients (75%) with HAT could be salvaged compared to only 25% patients in Group I (p=0.12). Incidence of portal vein thrombosis (PVT) has been remained similar (p=0.2) in the two eras.
Yang et al. (2014) found that independent risk factors associated with early HAT were recipient/donor weight ratio ≥1.15 (OR=4.499), duration of hepatic artery anastomosis >80 min (OR=5.429), number of units of blood received intraoperatively ≥7 (OR=4.059) and postoperative blood transfusion (OR=6.898). After logistic regression, duration of operation >10 h (OR=6.394), re-transplantation (OR=21.793) and rejection reactions (OR=16.936) were identified as independent risk factors associated with early HAT. Graft type (whole/living-donor/split), duration of operation >10 h, re-transplantation, rejection episodes, recipients with diabetes preoperatively and recipients with a high level of blood glucose or diabetes postoperatively had a higher risk of late HAT in the univariate analysis. Doppler exams of the hepatic artery and portal vein are frequently performed in the early postoperative setting. HAT in the early postoperative period can be managed with thrombectomy. Late HAT with complication of bile duct strictures is managed by interventional endoscopic retrograde cholangiography (ERC) but requires re-transplantation in the majority of patients. Early portal vein thrombosis is rare (<1%) but may lead to graft loss if not revascularised.
Primary non-functioning graft (PNFG) may be clinically obvious immediately after revascularisation of the allograft. Early signs of liver dysfunction include prolonged coagulation times, elevated liver enzymes (transaminases, cholestasis parameters) without a downward trend, rising lactate, and hypoglycemic episodes. PNFG is a critical situation and requires immediate re-transplantation.
Infections occurring during the first month post-LT are usually nosocomial, donor-derived, or due to perioperative complications (Hernandez 2015). Death within the first year after LT is often associated with bacterial infections. Management of infections due to multidrugresistant gram positive pathogens represents a major therapeutic challenge in the transplant setting (Radunz 2011).
Overall incidence of fungal infections in LT recipients has declined due to early identification and treatment of high-risk patients. However, overall mortality rate for invasive candidiasis and aspergillosis remains high (Liu 2011).
The clinical symptoms of early T-cell mediated rejection (TCMR) are non-specific, may not be apparent or may manifest as fever, right upper quadrant pain, and malaise. A liver biopsy is indispensable for confirming the diagnosis. High dose corticosteroids (3 days of 500-1000 mg methylprednisolone) are the first-line treatment for moderate and severe TCMR. A small study (n=28) by Volpin et al compares a high dose methylprednisolone schedule (1000mg for 3 consecutive days) to a lower dose protocol (single 1000mg of methylprednisolone followed by a 6-day taper from 200 to 20mg/day) (Volpin 2002). The treatment response was evaluated by a second liver biopsy. The taper protocol was more effective and safer that the 3 days high dose schedule and corticosteroid side effects were lower. In selected TCMR cases antibody-depleting therapy may be necessary. Mild, moderate and severe TCMR should be treated by an increase in CNI. Diagnosis of acute antibody-mediated rejection (AMR) requires a liver biopsy demonstrating classic histology and C4d+ staining (Demetris 2016). Mild AMR should be treated with steroid boluses. Moderate to severe cases can include plasmapheresis and intravenous immunoglobulins with or without anti-B cell agents. In contrast to late TCMR early TCMR (<6 weeks after LT) is not associated with reduced patient or graft survival after LT when treated adequately, but patients with moderate-to-severe early TCMR are at an increased risk for late TCMR (Jadlowiec 2019).
Subclinical TCMR (subTCMR) describes the presence of histological features of TCMR but without relevant elevation of liver enzymes. subTCMR is seen in up to 25% after liver transplantation and has a good short-term prognosis even without any specific therapy. There is no therapy needed if transaminases <2 ULN because there is no progression in fibrosis reported but immunosuppressive therapy should not be reduced. Positivity for donor-specific antibodies (DSA) in subTCMR is associated with an impaired graft and patient survival due to an upregulation of rejection associated transcripts (Höfer 2020).
Long-term complications after liver transplantation
Management issues for the long term include opportunistic infections, chronic ductopenic rejection, side effects due to immunosuppression including cardiovascular complications and renal dysfunction, de novo malignancies, biliary complications, osteoporosis and disease recurrence.
Opportunistic infections
Opportunistic infections in the medium and long term after LT are primarily viral and fungal in origin. Opportunistic bacterial infections are uncommon after 6 months in patients receiving stable and reduced maintenance doses of immunosuppression with good graft function. There is still a need for prospective interventional trials assessing the potential effects of preventive and therapeutic strategies against bacterial and fungal infection for reducing or delaying the development of chronic allograft dysfunction.
Cytomegalovirus (CMV) infection plays an important role in the LT setting (Mumtaz 2014) (Figure 2). CMV DNA assay is the commonly used laboratory tool to diagnose and monitor CMV infection. Current guidelines recommend antiviral prophylaxis over pre-emptive therapy in preventing CMV disease in high-risk LT recipients (CMV-seronegative recipients of organs from CMV-seropositive donors [D+/R-], [Kotton 2018]) as antiviral prophylaxis, compared with preemptive therapy, is superior in controlling CMV infections without an increased risk of rejection or opportunistic infections (Yadav 2022). The period of prophylaxis should be no shorter than 3 months in D+/R- patients. Delayed-onset CMV disease occurs in 15-38% of CMV D+/R- LT patients after prophylactic treatment for 3 months (Eid 2010, EASL 2016).
The procedure in the transplant centres is inconsistent for intermediate risk (R+) patients. If a preemptive strategy is adopted, screening for CMV every 1-2 weeks in the first 3 months post-LT is not entirely achievable in routine clinical practice in most centres. If prophylaxis is carried out in D+/R+ or D-/R+ patients, this should last 3 months. D-/R- patients have the lowest risk of CMV infection and disease.
A controlled clinical trial demonstrated that valganciclovir, an oral prodrug of ganciclovir, is as effective and safe as intravenouos (IV) ganciclovir for the prophylaxis of CMV disease in solid organ (including liver) transplant recipients (Paya 2004). In a published study by Kim et al. (2015) LT patients experiencing CMV infection were administered oral valganciclovir (900 mg/day) daily or IV ganciclovir (5 mg/kg twice daily) as antiviral preemptive treatment. A total of 83 patients had preemptive antiviral therapy, of those 61 patients received ganciclovir and 22 patients received valganciclovir. The median time from LT to CMV infection in the IV ganciclovir group was shorter than in the oral valganciclovir group (21 days vs. 30 days, p = 0.001). Recurrent CMV infection rates after treatment were 14.8% in the ganciclovir and 4.5% in the valganciclovir group (p=0.277). None of the patients in either group experienced CMV disease. The authors concluded that oral valganciclovir was equally effective as IV ganciclovir in preemptive treatment of CMV infection following LT.
Therapies for refractory CMV-infections are limited by toxicities. In 2022 Maribavir was authorised for patients after stem cell or solid organ transplantation with or without resistence. Maribavir is an oral antiviral medication and was superior to (val)ganciclovir for CMV viraemia clearance in the SOLSTICE trial (Avery 2022).
Occurrence of posttransplant lymphoproliferative disease (PTLD) in the first year after solid-organ transplantation is typically related to EpsteinBarr virus (EBV) infection. Incidence ranges between 3 and 21% (Choudhary 2021). EBV-seronegativity of the recipient before infection, high EB viral load, intensity of immunosuppression and young age have been reported as risk factors for PTLD (Smets 2002). Outcomes have improved since rituximab has been incorporated into treatment regimens (Kamdar 2011). Therapeutic management options include reduction of immunosuppression, rituximab, combination chemotherapy and adoptive immunotherapy. The use of CD19 chimeric antigen receptor T-cell (CAR-T) therapy for relapsed/refreactory PTLD is possible. A lately published retrospective multicentre study by McKenna et al showed an overall response rate of 64% with a two-year overall survival rate of 58% respectively (McKenna 2023).
Oral reactivation of human herpes simplex virus-1 (HSV-1) after LT is common. Development of varicella-zoster virus (HHV-3) after LT is typically related to intense immunosuppressive therapy and its therapy does not differ from the non-transplant setting. There is a vaccination against varicella-zoster virus. In Germany the vaccination with a dead vaccine is recommended from the age of 50 (Gross 2020).
Human herpesvirus 6 (HHV-6A and HHV-6B) can cause primary or reactive infection in LT recipients and may often be restricted to the infected organ and asymptomatic but it can also display a variety of clinical syndromes, including fever, hepatitis, and higher rates of graft dysfunction. It may have indirect effects including increased risks of mortality and fibrosis as well as hepatitis C progression. Recipients with inherited chromosomally integrated HHV-6 (ciHHV-6) may have an increased risk of graft rejection and opportunistic infections (Phan 2018). HHV-6 and HHV-7 may have a potential role as co-pathogens in the direct and indirect illnesses caused by CMV. HHV-6 infection can be determined by quantifying viral DNA in plasma or blood, however, biopsy remains the gold standard for diagnosis. Clinically significant tissue-invasive infections can be treated with ganciclovir, foscarnet or cidofovir.
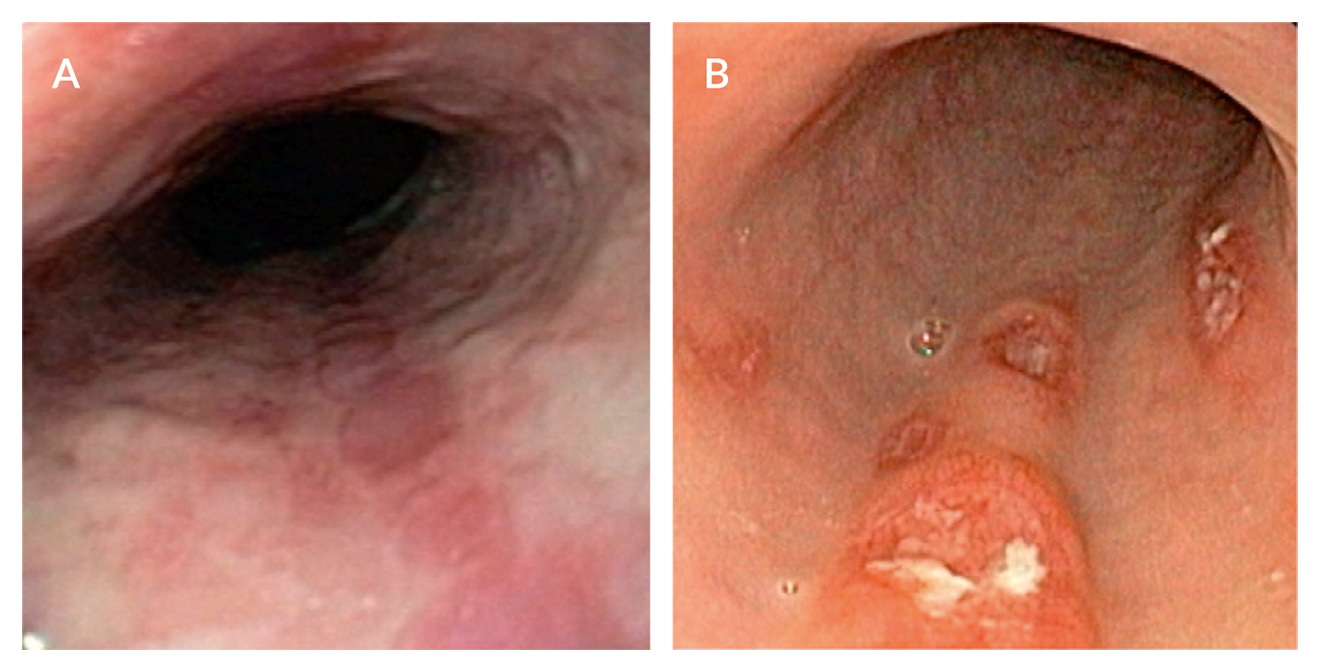 Figure 2. Cytomegalovirus (CMV) infection of the upper gastrointestinal tract. A. Livertransplanted patient complaining of dysphagia and epigastric discomfort with multiple longitudinal oesophageal ulcers seen at upper endoscopy. B. Endoscopic findings of deep oesophageal ulcerations with fibrinoid necrosis in another immunocompromised patient. In both cases, lesions were caused by CMV infection. Diagnosis depends on a positive mucosal biopsy, which should include specimens from the ulcer margins and ulcer base. Hematoxylin and eosin staining typically reveals “owl’s eye” cytoplasmic and intranuclear inclusion bodies.
Figure 2. Cytomegalovirus (CMV) infection of the upper gastrointestinal tract. A. Livertransplanted patient complaining of dysphagia and epigastric discomfort with multiple longitudinal oesophageal ulcers seen at upper endoscopy. B. Endoscopic findings of deep oesophageal ulcerations with fibrinoid necrosis in another immunocompromised patient. In both cases, lesions were caused by CMV infection. Diagnosis depends on a positive mucosal biopsy, which should include specimens from the ulcer margins and ulcer base. Hematoxylin and eosin staining typically reveals “owl’s eye” cytoplasmic and intranuclear inclusion bodies.
Hepatitis E
There is often a multifactorial pathogenesis for allograft hepatitis in LT patients. It is advisable to incorporate HEV RNA determination into the differential diagnostic investigation where patients have unexplained elevated liver enzymes or histological signs of allograft hepatitis (Borg 2016). Recently, molecular testing was suggested for HEV in transplant liver biopsies for evaluating patients with elevated transaminases of unknown origin (Protzer 2015).
Treatment of acute HEV infection with RBV may be indicated in specific cases of acute infection with severe liver dysfunction or extrahepatic manifestations. Chronic disease courses with HEV infections as well as reactivation after apparent cure have been reported in organ transplant patients. In the transplant setting, HEV Guidelines from UK (McPherson 2018) define diagnosis of persistent HEV infection leading to chronic hepatitis when HEV RNA is detectable in blood or stool for more than three months after the onset of relevant symptoms, raised liver enzymes, or from the first positive HEV RNA test.
The risk of HEV infection becoming chronic in immunocompromised (transplanted) patients is high, at around 60-65% (Kamar 2010a 2011, Legrand-Abravanel 2010, McPherson 2018). Quantification of HEV viral load is useful before initiation of antiviral therapy. HEV diagnosis should be bases on PCR techniques (Markakis 2022). A baseline quantitative HEV RNA assessment is undertaken on both plasma and stool at the start of treatment. A strong decrease of viral load may predict viral elimination.
A group from the Hannover Transplant Centre performed HEV serology tests in 226 LT patients, 129 non-transplanted patients with liver disease, and 108 healthy controls (Pischke 2010). HEV antibodies were detectable in 4% of the transplant group, 3% of the group with liver disease and 1% of the healthy control group. Three patients from the transplant group were HEV RNA positive, two of whom developed HEV viral persistence. Anti-HEV seroconversion was observed no earlier than four months after detection of HEV RNA.
The outcome, progression and individual variables associated with HEV infection becoming chronic were analysed in a retrospective study (Kamar 2011) including data from 17 transplant centres. The vast majority of the patients had received kidney (n=48) or liver (n=27) allografts. Chronification of HEV infection was defined as persistently elevated liver enzymes and positive detection of HEV replication in serum and/or feces over a minimum of six months. 65/85 patients (65.9%) developed a chronic disease. All 59 patients who underwent HEV genotyping had genotype 3. In contrast to the non-immunosuppressed patients, transaminases were usually only moderately elevated. Anti-HEV IgM was detectable in only 41% and IgG was detectable in 80.8%. 14.3% of the patients developed cirrhosis of the liver by the final follow-up.
In a recently published review of the literature sustained virological response was achieved by reduction of immunosuppression alone and by ribavirin regiments in 15% and 83% respectively (Markakis 2022).
With regard to PEG-interferon α treatment of HEV infection (Abbas 2014, Kamar 2010c), there is little data available for LT patients and this treatment approach should not be used as first line therapy. HEV RNA testing in plasma and stool at day 7 and monthly after RBV treatment initiation is recommended. A 3-month course of RBV monotherapy seems to be an appropriate treatment duration if stool tests are negative for HEV RNA at month 3 on two occasions (McPherson 2018). If HEV RNA is positive at month 3, RBV is continued until stool tests are negative for HEV RNA on two occasions one month apart or RBV is continued for 6 months. A test of SVR is conducted by testing plasma and stool samples for HEV RNA at three and six months after cessation of antiviral therapy.
Chronic rejection (TCMR and AMR)
Advances in immunosuppressive regimens have greatly reduced the incidence of chronic rejection and allograft failure. Chronic rejection begins within weeks to months or years after LT and accounts for a small proportion of late graft dysfunction (Suhling 2016). It affects about 4% to 8% of patients (Neuberger 1999).
Sub-therapeutic immunosuppression and nonadherence to immunosuppressive therapy also coincides with increased risk of rejection, substantial increases in the rates of graft loss and death. Special attention should be posed on immunosuppression-related physical side effects as a major reason for non-adherence. Multidisciplinary evaluation, in particular by transplant hepatologists and psychologists are warranted to improve adherence before and after LT. Chronic TCMR and AMR may appear indolently and might only become apparent as liver test injury abnormalities (GGT, AP, bilirubin, transaminases). The diagnosis needs to be confirmed by histopathologic examination. Chronic TCMR results in potentially irreversible bile duct and vascular injury. Treatment is difficult. Patients on cyclosporine (CSA) should be switched to tacrolimus (TAC). Diagnosis of chronic AMR includes inflammation with low grade interface activity, fibrosis and C4d+ staining (Demetris 2016). There is currently no defined treatment strategy. Switching the baseline immunosuppression from CSA to TAC and initiating mycophenolate mofetil (MMF) rescue therapy represents a treatment option in these patients (Daly 2002).
Calcineurin inhibitor-induced nephrotoxicity and alternative immunosuppressive protocols
Despite the introduction of new immunosuppressive agents (Table 4), calcineurin inhibitors (CNI) remain the key drugs in most immunosuppressive regimens. Both CSA and TAC inhibit the calcineurincalmodulin complex and therefore IL-2 production in T lymphocytes. TAC is available as traditional twice-daily immediate-release tacrolimus and once-daily prolonged/extended released formulations. Renal failure, mainly due to CNI nephrotoxicity, is the most common complication following orthotopic LT. The incidence of chronic renal dysfunction characterised by arteriolar hyalinosis resulting in a variety of tubulointerstitial and glomerular lesions has been reported in up to 70% of patients in the long term after LT and varies widely depending on the length of follow-up, the definition of chronic kidney disease and the intensity of immunosuppressive therapy (Beckebaum 2013b). End stage renal disease has been described in 18% of patients during a posttransplant follow-up of 13 years (Gonwa 2001).
Randomised trials have shown that induction therapy maintains immunosuppressive efficacy in steroid-free regimens. For instance, delayed CNI initiation (e.g. to days 4-5 posttransplant) can prevent deterioration of renal function posttransplant, but requires induction with an interleukin-2 antagonist receptor (IL-2RA) agent or rabbit antithymocyte globulin (rATG) to maintain early immunosuppressive efficacy.
A group from Regensburg initiated a single arm pilot study to determine the safety and efficacy of a CNI-free combination therapy (basiliximab induction/MPA and delayed [10 days posttransplant] SRL in patients with impaired renal function (GFR <50 mL/min and/or serum creatinine >1.5 mg/ dL) at LT (Schnitzbauer 2015). Renal function improved significantly (p = 0.006). The critical time period for relevant improvement of kidney function seemed to be the first month, independently from SRL administration.
In LT patients with CNI-induced nephrotoxicity, a complete replacement of CNI with conversion to MMF has shown conflicting results with respect to the occurence of rejection, anywhere from 0% to 60% (Creput 2007, Schmeding 2011, Moreno 2004). MMF inhibits inosine monophosphate dehydrogenase, a critical enzyme in the de novo pathway of purine synthesis. Results from previous studies with immunosuppressive regimens including MMF and minimal CNI treatment suggest a significant improvement in renal function in this patient group (Beckebaum 2011, Cicinnati 2007a, Beckebaum 2004a, Cantarovich 2003, Garcia 2003, Raimondo 2003).
De novo immunosuppression with MMF combined with induction therapy and delayed CNI introduction is another approach to reduce CNI related nephrotoxicity especially in patients with higher MELD score or significant renal dysfunction. In a randomised clinical trial, a daclizumab/ MMF/delayed low-dose TAC-based regimen was compared with a standard TAC/MMF regimen (Yoshida 2005). In both study arms, corticosteroids were tapered over time. Statistically significant higher median GFR was found in the delayed CNI group, although acute rejection episodes were not statistically significant different between the groups. Similar results were seen in two retrospective studies in LT patients receiving thymoglobulin induction therapy and a delayed initiation of CNI (Bajjoka 2008, Soliman 2007).
Another approach to maintain renal preservation is replacement of CNI by mTOR inhibitors such as SRL or everolimus (EVL) (Sanchez 2005, Harper 2011, Kawahara 2011, Hüsing (a) 2015) particularly in HCC-patients due to antitumour effects.
An Italian consensus Transplant panel even recommended routine use of EVL in predefined clinical scenarios, particularly in light of posttransplant nephrotoxicity (de Simone (a) 2016).
In the multicentre randomised (1:1) controlled PROTECT study (CRAD001HDE10) de novo patients were treated with CNI (CSA or TAC) + basiliximab ± steroids for 4-8 weeks after LT and were then randomised to an EVL-based treatment arm or a CNI-based control arm (Fischer 2012). In the EVL-based treatment arm (n=101), a 70% reduction of CNI (± steroids) was carried out over a period of 2 months, followed by treatment with EVL ± steroids. In the control arm (n=102) treatment with CNI (standard dose ± steroids) was continued. Using the MDRD equation, the endpoint could be achieved with a difference in calculated GFR of at least 8 mL/min between the two treatment arms (p=0.02). The incidence of graft rejection, graft loss and death were not significantly different between the two treatment arms. A 24-month extension phase was performed in 81 patients to month 35 post-randomisation. The adjusted mean eGFR benefit from randomisation to month 35 was 9.4 mL/min/1.73 m2 with MDRD. The difference in favour of the CNI-free regimen increased gradually over time due to a small progressive decline in eGFR in the CNI group (Sterneck 2014).
A study by Hanover transplant centre outlined that a surveillance biopsy guided personalised immunosuppression programme leads to immunosuppression reduction and a significantly better kidney function (Saunders 2021).
Efficacy and safety of a TAC-free and a TAC-reduced regimen were compared with a TAC standard dose (TAC-C) regimen in a multinational, randomised controlled licensing trial (CRAD001H2304) in de novo LT recipients (Saliba 2011b). After a 1-month run-in phase on TAC-based immunosuppression (+/-MMF), patients were randomised to an EVL/ prednisone/TAC-free group (TAC-WD) including TAC withdrawal at 4 months post-LT, an EVL/prednisone/reduced TAC group (EVL+rTAC) or a standard TAC control group (TAC-C). The primary combined endpoint included biopsy-confirmed acute rejection, allograft loss or death, and the secondary endpoint was renal function at 1 year. The TAC-WD arm was stopped prematurely due to a significantly higher incidence of biopsyconfirmed acute rejections (19.9% [TAC-WD] vs. 4.1% [EVL+rTAC] vs. 10.7% [TAC-C]).
At 1 year, significantly more patients in the TAC-C group had reached the combined primary endpoint compared to the EVL+rTAC group (9.7% vs. 6.7%; p<0.001). Kidney function was significantly better (p<0.001) in the EVL+rTAC arm than in the TAC-C arm. The increased rejection rate in the TAC-WD group at month 4 may be caused by the immunosuppressive strategy used. Unlike the CRAD001HDE10 study, no induction therapy with an anti-IL-2 inhibitor was performed and there was no weaning of CNI over 2 months. Instead, CNI were stopped abruptly.
Lin (2016) conducted a systematic review and meta-analysis of randomised controlled trials (RCT) analysing the effect of EVL on renal function in patients (EVL n=465, control n=428) with baseline GFR >30 mL/min undergoing a CNI minimisation or withdrawal protocol. Based on these results, EVL use with CNI minimisation in LT recipients was associated with improved renal function at 12 months (95% CI 2.75-17.8) but not associated with an increased risk of biopsy proven acute rejection (RR 0.68, 95% CI 0.31-1.46), graft loss (RR 1.60, 95% CI 0.51-5.00), or mortality (RR 1.34, 95% CI 0.62-2.90). However, it was associated with an increased risk of overall infections (RR 1.45, 95% CI 1.10-1.91).
In the randomised controlled multicentre SiLVER trial the per protocol analysis identified LT recipients with early CNI minimisation and introduction of SRL within 4 to 6 weeks after LT with significantly superior eGFR and lowest rate of chronic kidney disease (≥ stage 3) from year 1 during a follow-up period of 5-years (Buchholz 2020).
Early institution at one month of EVL in combination with low dose TAC (≤5 ng/mL) for preserving kidney function has also been recommended by the International Liver Transplant Society Consensus guidelines on immunosuppression in LT recipients (Charlton [c] 2019).
In future, there might be further development of cell therapeutic approaches and mesenchymal stem cells to launch tolerogenicity rather than development of new immunosuppressive drugs (Charlton [c] 2019).
Table 4. Clinically used immunosuppressive agents in liver transplantation| Immunosuppressant class | Immunosuppressive agent |
| Corticosteroids | Prednisone, prednisolone, methylprednisolone |
| Calcineurin inhibitors | Cyclosporin, tacrolimus |
| Antimetabolites | Mycophenolate mofetil, azathioprine |
| mTOR Inhibitors | Sirolimus, everolimus |
| Polyclonal antibodies | Antithymocyte globulin |
| Monoclonal anti-CD3 antibodies | Muromonab-CD3 (OKT3) |
| Chimeric monoclonal antibodies | Anti-IL-2 receptor inhibitor (basiliximab) |
| Monoclonal anti-CD52 antibodies | Alemtuzumab (campath-1H) |
Other side effects of CNI
Besides potential nephrotoxicity, CNI therapy is associated with side effects that include cardiovascular complications, tremor, headache, electrolyte abnormalities, hyperuricaemia, hepatotoxicity, and gastrointestinal symptoms. Neurotoxicity, including tremor, paresthesia, muscle weakness, and seizures, more often occurs in TAC-treated patients; gingival hyperplasia, a rare event, and hirsutism are associated with CSA treatment.
Cardiovascular side effects due to CNI and steroids include hyperlipidaemia, arterial hypertension, and diabetes (Beckebaum 2004b).
The prevalence of new-onset diabetes mellitus after LT has been reported
to occur in 9-21% of patients (John 2002, Konrad 2000). The prevalence of posttransplant diabetes is even higher if cofactors such as hepatitis C are present. In various studies, the diabetogenic potential has been reported to be higher in patients receiving TAC than in those receiving CSA. In contrast, CSA has a more pronounced effect on lipid levels. CSA can act by modulating the activity of the LDL receptor or by inhibiting the bile acid 26-hydroxylase that induces bile acid synthesis from cholesterol.
Numerous studies aimed to determine the most effective immunosuppressive protocols while minimising drug-related side effects. These protocols often combine several drugs with different mechanisms of action and toxicities allowing dose adjustment. There is also a trend towards tailored immunosuppressive regimens following the aetiology of liver disease and comorbidities such as renal dysfunction and cardiovascular disease
A systematic review by Bzeizi et al including eight studies with 769 patients compared Everolimus alone or in combination with reduced CNI dose and showed a better renal function in patients with reduced CNI dose levels (Bzeizi 2021). A better long-term renal outcome was also shown for selected LT patients with Sirolimus-based immunosuppression and CNI reduction (Buchholz 2020).
Corticosteroid minimisation/avoidance protocols and additional strategies to reduce metabolic complications
There is ongoing discussion of steroid avoidance due to dyslipidaemia, osteoporosis, development of cataracts, weight gain, hypertension, and a deleterious impact on glucose control. As cardiovascular disease is the second leading cause of death in the late transplant period, steroidminimised/free regimens may be favoured in particular in patients with high risk of metabolic syndrome.
A metaanalysis including 16 studies with 1347 participants showed that glucocorticosteroid avoidance or withdrawal appears to reduce diabetes mellitus and hypertension (Fairfield 2018). In a study, Yoo et al. (2015) evaluated outcomes of 500 consecutive LT recipients who received a steroid-free protocol with rATG induction and a single dose of methylprednisolone given before the first dose of rATG. Mean MELD at transplantation was 22 ± 6. MMF was initiated postoperatively with delayed TAC initiation at 4.79 ± 13.3 days. TAC was replaced by SRL if serum creatinine remained above 2.0 mg/dL after 1 week. Patients were switched to TAC or SRL monotherapy at 12 weeks. Posttransplant peak creatinine was at 1 month 1.43 ± 0.95 mg/dL and improved to 1.26 ± 0.60 mg/dL (p< 0.05) at 2.5 years. Lowest GFR rate was observed at 1 month (65.6 ± 30.0) and improved by 12 months (72.7 ± 28.2, p< 0.01). One-year patient and graft survival were 92.8% and 89.6%, respectively. Rejection occurred in 22.8% of patients, 6.6% of patients had steroid-dependent rejection.
Other research groups have reported encouraging findings with steroidfree protocols including basiliximab induction therapy (Filipponi 2004, Llado 2008, Becker 2008). In a multicentre, 24-week, randomised, open-label, phase IIIb trial (DIAMOND study) renal function was investigated with once-daily, prolonged-release TAC-based immunosuppression in de novo LT recipients. Patients were administered prolonged-release TAC (initial dose 0.2 mg/kg/day); prolonged-release TAC (0.15-0.175 mg/kg/day) plus basiliximab or prolonged-release TAC (0.2 mg/kg/day delayed until Day 5) plus basiliximab. All patients had comedication with MMF plus a bolus of corticosteroids. Lower dose prolonged-release TAC (0.15–0.175 mg/kg/day) immediately posttransplant in combination with basiliximab and MMF was associated with lower TAC exposure, significantly reduced renal function impairment and biopsy-confirmed acute rejection incidence vs. prolongedrelease TAC (0.2 mg/kg/day) administered immediately after LT. Delayed higher-dose prolonged-release TAC exposure significantly reduced renal impairment compared with immediate administration (Trunecka 2015).
A published literature review (Lerut 2009) analysed the actual status of corticosteroid minimisation protocols in LT based on a detailed analysis of 51 peer-reviewed and 6 non-peer-reviewed studies. Results from the majority of studies showed that these protocols have clear metabolic benefits and are safe with respect to graft and patient survival. These results are in line with a recent metaanalysis of 16 studies with 1347 participants demonstrating that metabolic complications such as diabetes and hypertension were statistically significantly less frequent in patients undergoing steroid avoidance or withdrawal protocols vs. steroidcontaining immunosuppression (Fairfield 2018).
A healthy diet and regular exercise represent additional effective strategies to avoid or reduce serious cardiovascular complications. In patients with dyslipidaemia, hydrophilic statins such as pravastatin and fluvastatin should be preferred as they are not metabolised by cytochrome P450–3A4.
De novo malignancies
Incidence of malignancies is higher in transplant patients and depends on the length of follow-up, characteristics of the transplant population, choice of immunosuppressive therapy and the era when the LT was performed (Buell 2005, Fung 2001). A cumulative risk has been reported of 10%, 24%, 32% and 42% at 5, 10, 15 and 20 years, respectively, for development of de novo cancers after LT (Finkenstedt 2009). The highest risks in the transplant setting are non-melanoma skin cancers (21.7%) (Saglam 2022), mainly squamous cell carcinoma and basal cell carcinoma (Figure 3). Regular cancer surveillance programmes have been proposed by several groups; however, scientific evidence is lacking and surveillance programmes may vary from centre to centre.
Bhat et al. (2018) investigated potential risk factors for malignancies after
LT analysing data from the Scientific Registry of Transplant Recipients database comprising 108, 412 LT recipients. During median follow-up of 6.95 years malignancies during follow-up were 4, 483 (41.3%) skin, 1, 519 (14.0%) hematologic, and 4, 842 (44.7%) solid organ. The 10-year probability of de novo malignancy was 11.5% (11.3-11.8%). Multivariable analysis showed that age by decade, male gender, Caucasian race, multiorgan transplant, previous malignancy and alcohol-related, autoimmune-related, and NASH-related liver disease and PSC pre-LT (compared to HCV, p<0.001) were associated with higher risk of post-LT malignancy. There was no correlation between type of immunosuppression and risk of cancer. Findings were confirmed by Launoy et al (Launoy 2021).
Patients with replicative EBV infection and immunosuppressive regimens, i.e. ATG, are at a higher risk of developing PTLD. These patients may present with lymphoadenopathy and/or fever, weight loss and night sweats, and meticulous examination, serologic and imaging tests are required. Diagnosis and classification of PTLD is currently based on histologic criteria, and a multidisciplinary team is required including hematologists and transplant hepatologists for treatment of PTLD, monitoring of immunosuppressive therapy and preservation of allograft function.
In a prospective single-centre study the relationship between the development of solid organ cancers following LT and the level of CNI exposure was assessed (Carenco 2015). Data are based on 247 TAC-treated LT recipients who survived at least 1 year posttransplant. Study results showed that 43 (17.4%) patients developed de novo solid cancers. Mean TAC concentration during the first year after LT was significantly higher in patients who developed solid malignancies (10.3 ± 2.1 vs. 7.9 ± 1.9 ng/mL, p < 0.0001). Independent risk factors in multivariate analysis were tobacco consumption pretransplant (OR = 5.42; 95% CI [1.93-15.2], p = 0.0014) and mean annual TAC concentration during the first 12 months posttransplant (p < 0.0001; OR = 2.01; 95% CI [1.57-2.59], p < 0.0001). Similar results have been shown in a subgroup of patients exposed to TAC continuously for ≥3 years. Premalignant lesions such as actinic keratoses are mostly located on sun-exposed areas. Squamous cell carcinoma and basal cell carcinoma are increased by factors of ~65-200 and ~10, respectively, in organ transplant recipients as compared to the immunocompetent population (Ulrich 2008). An annual routine dermatologic follow-up exam, limitation of sun exposure and protective measures including sunscreens are highly recommended for transplant patients. Due to a higher incidence of colon cancer in patients transplanted for PSC and concomitant inflammatory bowel disease (Hanouneh 2011) an adequate colonoscopic surveillance is required at regular intervals (annual colonoscopy) even in the absence of active disease (Fevery 2012). A trend has recently been reported toward an increased incidence of advanced colon polyps and colon carcinoma in patients transplanted for diseases other than PSC after LT. However, larger studies are needed to determine whether posttransplant colon cancer surveillance should be performed more frequently than in the non-transplant setting (Rudraraju 2008).
Studies have reported a significantly higher incidence of aerodigestive cancer including lung cancer among patients who underwent LT for alcohol-related liver disease (Vallejo 2005, Jimenez 2005). These patients should undergo a more intensive surveillance protocol for the detection of upper gastrointestinal and oropharyngeal-laryngeal malignancies (Benlloch 2004). In cases of positive smoking history surveillance for lung cancer should be implemented. In a retrospective study, conversion from CNI to an mTOR inhibitor (EVL) improved the prognosis of de novo malignancies after LT for alcoholic cirrhosis (Thimonier 2014). One- and five-year survival was 77.4% and 35.2% in the EVL cohort vs. 47.2% and 19.4% in the non-EVL cohort, respectively (p=0.003).
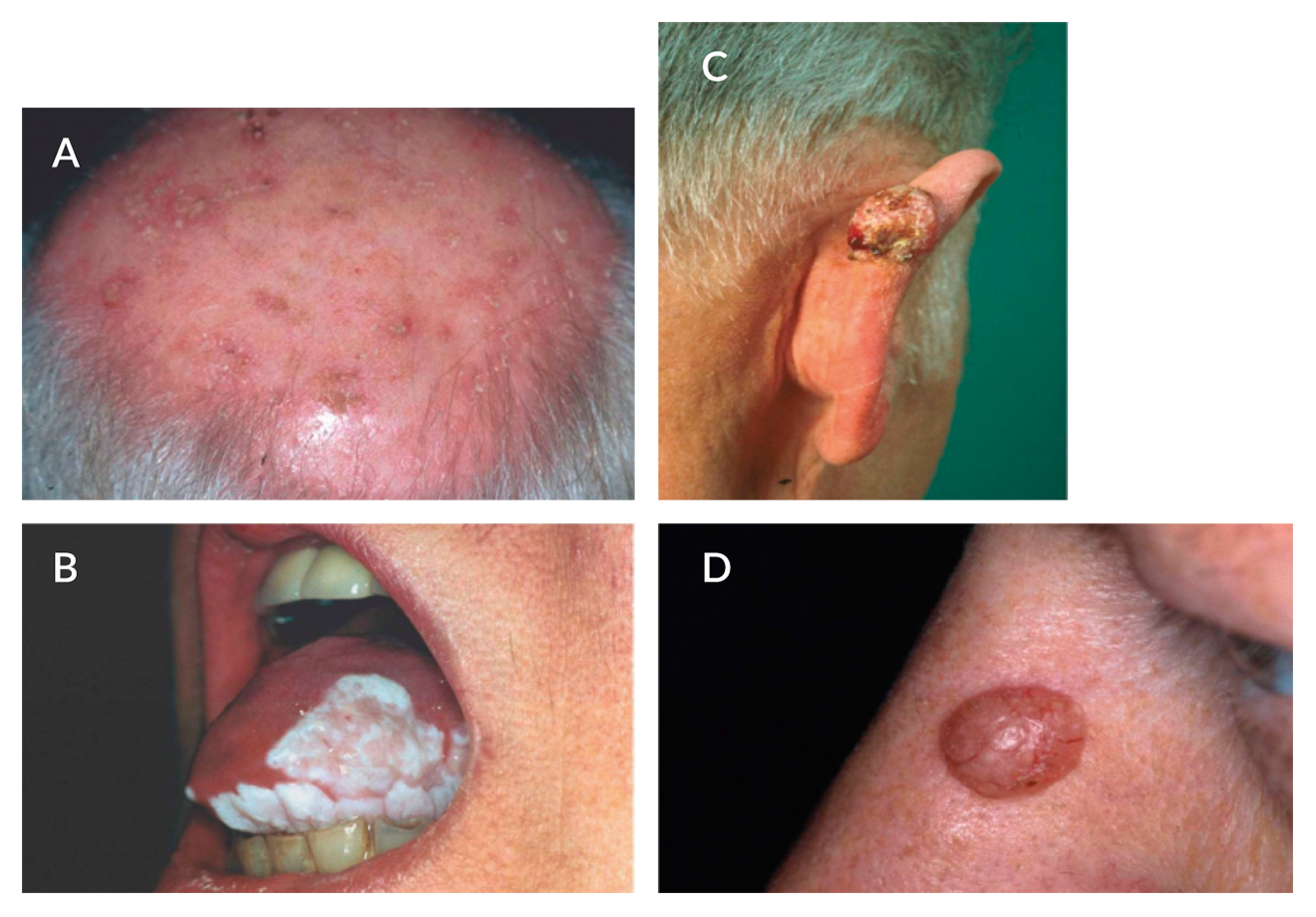 Figure 3. Non-melanoma skin scancers and liver transplantation (LT). Organ transplant recipients have an increased risk of development of non-melanoma skin cancers as compared to the non-transplant setting. Premalignant lesions such as actinic keratoses [A] are predominantly located on sun-exposed areas. Squamous cell carcinoma [B, C] is the most frequent skin cancer after LT followed by basal cell carcinoma [D] (Photographs kindly provided by Prof. Dr. Hillen, Transplant Dermatology Outpatient Unit, Department of Dermatology, University Hospital Essen, Germany)
Figure 3. Non-melanoma skin scancers and liver transplantation (LT). Organ transplant recipients have an increased risk of development of non-melanoma skin cancers as compared to the non-transplant setting. Premalignant lesions such as actinic keratoses [A] are predominantly located on sun-exposed areas. Squamous cell carcinoma [B, C] is the most frequent skin cancer after LT followed by basal cell carcinoma [D] (Photographs kindly provided by Prof. Dr. Hillen, Transplant Dermatology Outpatient Unit, Department of Dermatology, University Hospital Essen, Germany)
Studies have shown that mTor inhibitors (SRL, EVL) exert antiangiogenic activities that are linked to a decrease in production of vascular endothelial growth factor (VEGF) and to a markedly inhibited response of vascular endothelial cells to stimulation by VEGF (Guba 2002). Furthermore, the ability of mTor inhibitors to increase the expression of E-cadherin suggests a mechanism for blocking regional tumour growth and for inhibiting metastatic progression. Therefore, we give special consideration for mTOR inhibitor-based immunosuppressive regimens not only in patients transplanted for HCC (Kang 2021) but also those with de novo malignancies after LT. There is evidence from meta-analyses and studies performed mainly in the kidney transplant setting that switching from CNI to mTOR-based immunosuppression is associated with a lower incidence of non-melanoma skin cancers (Euvrard 2012, Caroti 2012, Gu 2012, Adelmalek 2012). A multicentre study involving CNI-treated patients with a previous history of at least one squamous cell carcinoma randomly allocated patients to an arm in which CNI was replaced by SRL, or to an arm in which the CNI-based immunosuppression was continued (Euvrard 2012). The squamous cell carcinoma-free survival was significantly longer in the SRL group than in the CNI control group. The authors concluded that SRL obviously has an antitumour effect regarding the reappearance or the new appearance of non-melanoma skin cancers.
Biliary complications
The clinical outcome of patients posttransplant can be significantly affected by biliary complications (Lisotti 2015). Biliary leaks generally present as an early posttransplant complication and occur in 5% to 10% of deceased donor LT (Kapoor 2015) and in 10% to 15% of LDLT (Iida 2010). Biliary leaks are typically treated with placement of a biliary stent to bridge the leak, usually with sphincterotomy. In patients with biliary stones, endoscopic sphincterotomy and stone extraction are the treatment of choice. Biliary stone disease and in particular formation of biliary casts is common in the setting of LT and may occur without or in the setting of strictures due to impaired biliary flow. The exact aetiology of biliary cast disease is unknown but ischaemia and strictures have been described as predisposing factors (Pereira 2018). In a retrospective study complication rate during the first 15 days after endoscopic sphincterotomy were assessed in patients who underwent conventional or precut endoscopic sphincterotomy (Hüsing (b) 2015). A total of 24 complications (15.2%) were reported, including pancreatitis, bleeding, and perforation. Complication rates were not significantly different between the two sphincterotomy techniques.
Damage (ischaemia, infectious complications or rejection) of the biliary tree mucosa can provoke cast which consists of desquamated epithelial cells mixed with bile products within the biliary system and occurs in 3% to 18% of LT patients (Shah 2003).
Biliary strictures are one of the most common complications after LT, with a reported incidence of 5.8-34% (Graziadei 2006). Early anastomotic strictures usually have a technical origin, while strictures appearing later have a multifactorial origin. Non-anastomotic strictures without underlying hepatic artery thrombosis are commonly referred to as ischemic-type biliary lesions (ITBL). Hypothermic oxygenated machine perfusion led a lower risk of non-anastomotic strictures cardiac death (van Rijn 2021).
Risk factors for ITBL include preservation-induced injury, prolonged cold and warm ischaemia times, altered bile composition, ABO blood incompatibility and immunologic injury (Verdonk 2007, Buis 2009). A german transplant group found that specific chemokine receptor polymorphisms of the recipient are associated with the development of post-LT biliary strictures (Iacob 2012). Moreover, screening of anti-HLA antibodies might be useful for early identification of at-risk patients who could benefit from closer surveillance and tailored immunosuppressive regimen (Iacob 2012).
ERC or percutaneous transhepatic cholangiography (PTC) have typically been used as the primary approach, leaving surgical intervention for those who are non-responsive to endoscopic interventions or who have diffuse intrahepatic bile duct damage. Radiological methods such as magnetic resonance cholangiopancreatography (MRCP) have been introduced as an additional diagnostic tool for biliary complications. In cases of biliary cast and ischemic cholangiopathy, endoscopic ultrasound (EUS) was found to be diagnostically superior to ERCP and had a significant impact on clinical decision-making. EUS was less reliable when diagnosing anastomotic strictures (Hüsing 2015). EUS can complement ERCP to improve diagnosis of biliary complications after LT and impact on treatment decision.
The long-term efficacy and safety of endoscopic techniques have been evaluated in various transplant centres (Qin 2006, Zoepf 2012, Pascher 2005). Non-anastomotic strictures are commonly associated with a less favourable response to interventional endoscopic therapy in comparison to anastomosis stenosis (Figure 4). An Austrian group found anastomotic strictures in 12.6% of patients transplanted between October 1992 and December 2003 and non-anastomotic strictures in 3.7% during a mean follow-up of 53.7 months after LT (Graziadei 2006). Interventional endoscopic procedures were effective in 77% of patients with anastomosis stenosis, while treatment of non-anastomotic strictures showed long-term effectiveness in 63% of patients. A surgical approach was required in 7.4% of transplant recipients.
 Figure 4. Biliary tract complications after liver transplantation. A. Endoscopic retrograde cholangiography (ERC) showing posttransplant short filiform anastomotic biliary stricture in a 46-year-old patient transplanted for hepatitis C virus (HCV) infection and alcohol-related cirrhosis 6 months earlier. Therapy sessions include dilatation and an increasing number of bile duct endoprostheses at short intervals of every 2-3 months. Prior to endoscopic therapy an endoscopic sphincterotomy is performed. B. ERC of a 41-year-old patient transplanted for HCV diagnosed with ischemic-type biliary lesions (type 3) with long non-anastomotic stricture extending proximally from the site of the anastomosis and strictures throughout the entire liver.
Figure 4. Biliary tract complications after liver transplantation. A. Endoscopic retrograde cholangiography (ERC) showing posttransplant short filiform anastomotic biliary stricture in a 46-year-old patient transplanted for hepatitis C virus (HCV) infection and alcohol-related cirrhosis 6 months earlier. Therapy sessions include dilatation and an increasing number of bile duct endoprostheses at short intervals of every 2-3 months. Prior to endoscopic therapy an endoscopic sphincterotomy is performed. B. ERC of a 41-year-old patient transplanted for HCV diagnosed with ischemic-type biliary lesions (type 3) with long non-anastomotic stricture extending proximally from the site of the anastomosis and strictures throughout the entire liver.
Results from 75 transplanted patients undergoing ERC for suspected anastomotic strictures were retrospectively analysed (Zoepf 2006). Balloon dilatation alone and combined dilatation and endoprosthesis placement was efficacious in 89% and 87% of cases respectively, but recurrence occurred in 62% and 31% of cases respectively. However, results of these strategies are inconsistent in the literature. Repeated ERC sessions are commonly performed with increasing endoprosthesis diameter every three months and double or triple parallel stenting in selected cases. Up to 75% of patients are stent-free after 18 months of endoscopic intervention (Tung 1999).
Medical treatment for bile duct strictures consists of ursodeoxycholic acid (UDCA) and additional antibiotic treatment in stricture-induced cholangitis. Complications related to bilioenteric anastomosis require PTC or surgical intervention.
Metabolic bone disease
Liver cirrhosis, heavy alcohol use, smoking, poor nutrition, hypogonadism, cholestatic liver disease, and therapy with corticosteroids, older age, lower-pre-L BMI are risk factors for the development of osteoporosis in pretransplant patients (Schreiber 2018, Lim 2021). In a study assessing both vertebral and nonvertebral (rib, pelvic, and femur) fractures in pretransplant patients with PBC and PSC, 20% and 1, 4% of the patients had experienced fracturing and avascular necrosis, respectively (Guichelaar 2007). Screening with bone densitometry using dual-energy x-ray absorptiometry should begin prior to LT (Wibaux 2011).
A further increase in bone turnover has been described after LT going along with bone density decrease within the first 3 to 6 months after transplant. Bone density gradually returns to pretransplant levels thereafter (Singh 2015). Posttransplant bone disease contributes significantly to patients’ morbidity and mortality after transplantation and plays a role for their quality of life (Nel 2016). Factors favouring spinal bone gain from 4 to 24 months after transplantation include lower baseline and/or 4-month bone density, premenopausal status, lower cumulative glucocorticoids, no ongoing cholestasis, and higher levels of vitamin D and parathyroid hormone (Guichelaar 2006). CNI administration is a risk factor for osteoporosis after LT (Moreira Kulak 2010).
The risk of osteoporotic vertebral and nonaxial fractures was 14% and 21% at 1 and 2 years posttransplant, decreased with time, and was highest in patients with pretransplant osteopenia and cholestatic liver disease (Singh 2015).
A cumulative incidence of fractures at 1 year and at 8 years posttransplant was reported in 30% and 46% of patients transplanted for PBC and PSC (Guichelaar 2007). Nine percent experienced avascular necrosis after LT. This event was positively correlated with pretransplant and posttransplant lipid metabolism, bone mineral density and fracturing, and posttransplant glucocorticoid administration (Guichelaar 2007).
EASL Clinical Practice Guidelines focusing on Liver Transplantation (http://dx.doi.org/10.1016/j.jhep.2015.10.006) recommends bone mineral density screening yearly for patients with pre-existing osteoporosis and osteopenia, every 2-3 years in patients with normal bone mineral density and further screening intervals depending on impairment of bone mineral density and on risk factors. Regular bone mineral density screening may be hampered in some countries as it is not necessarily covered by (statutory) health insurances. There are no specific therapies for posttransplant osteoporosis besides those for non-transplanted patients. General interventions to reduce fracture risk include adequate intake of calcium and vitamin D. Secondary hyperparathyroidism and adverse lifestyle factors should be addressed and corrected. Bisphosphonates are currently the most effective agents for treatment of posttransplant osteoporosis (Moreira Kulak 2010) (www.dv-osteologie.org). A meta-analysis and systematic review of randomised controlled trials demonstrated that bisphosphonate therapy in the first 12 months post-LT is associated with reduced accelerated bone loss and improved bone mineral density at the lumbar spine (Kasturi 2010).
Recurrent diseases after liver transplantation
Disease recurrence may occur in patients transplanted for viral hepatitis, tumour disease, autoimmune or cholestatic or alcohol-related liver diseases.
Recurrence of hepatitis B in the allograft
HBV recurrence using combined prophylactic regimens is less than 5%. However, recurrence rates differ among various studies as most of them are small, with varying proportions of patients with active viral replication at LT and varying follow-up periods after LT. Combined use of hepatitis B immunoglobulin (HBIG) and nucleos(t)ide analogs (NUC) has emerged as treatment of choice in transplanted HBV recipients (Figure 5) (Cai 2012) and its efficacy has been investigated extensively. There is a high variability (dose, duration and method of HBIG administration) in the prophylactic protocols. According to the German guidelines (Cornberg 2021) patients receive 10, 000 IU HBIG IV in the anhepatic phase followed by 2000 IU a day during the first posttransplant week and 1000-2000 IU a month in the first year after LT. For long-term HBIG prophylaxis, trough anti-HBs levels at or above 100 IU/L should be maintained. For LT-patients with hepatitis B and D coinfection combined regime should be administered for a longer period (Orfanidou 2021).
The European Commission granted a marketing authorisation valid throughout the European Union for subcutaneous (SC) HBIG in 2009, and it has been launched in the last years in many European countries. SC HBIG application has advantages over intramuscular (IM) and IV administration (Yahyazadeh 2011, Beckebaum 2012, Beckebaum 2013c). It can be performed by patients at home, which is an important factor in improving patients’ flexibility and mobility in daily life, lowering the frequency of physician consultations and avoiding AEs attributable to high peak and low trough serum anti-HBs levels compared with IV administration (Yahyazadeh 2011, Beckebaum 2012, Beckebaum 2013c).
De Simone et al. (b) (2016) demonstrated that early introduction of SC HBIg administration by week 3 posttransplantation, combined with HBV virostatic prophylaxis, is safe and effective for prevention of HBV reinfection.
Data from a retrospective study including 371 adults transplanted for HBV-related disease at 20 European centres and treated with IV HBIG (n=299), SC HBIG (n=236), and other HBIG preparations for 12 months ± NUC therapy were analysed (Beckebaum 2018). The majority (93.5%) received NUC therapy. Recurrence was 16/371 (4.3%) (annual rate 0.65%); 5/16 patients with recurrence had discontinued HBIG and 7/16 had low anti-HBs titre (<100 IU/L). The recurrence rate in HBIG-treated patients was 1 per 2069 months. Risk of HBV recurrence in patients who discontinued HBIG was increased by 5.2-fold as compared to those on SC HBIG therapy.
Economic issues have led to a conduct of studies investigating whether NUC therapy instead of combined long-term NUC/HBIG is sufficient for antiviral prophylaxis (Cholongitas 2014, Teperman 2013, Buti 2007, Angus 2007, Knighton 2013, Gane 2007, Stravitz 2012, Wesdorp 2012, Fung 2011).
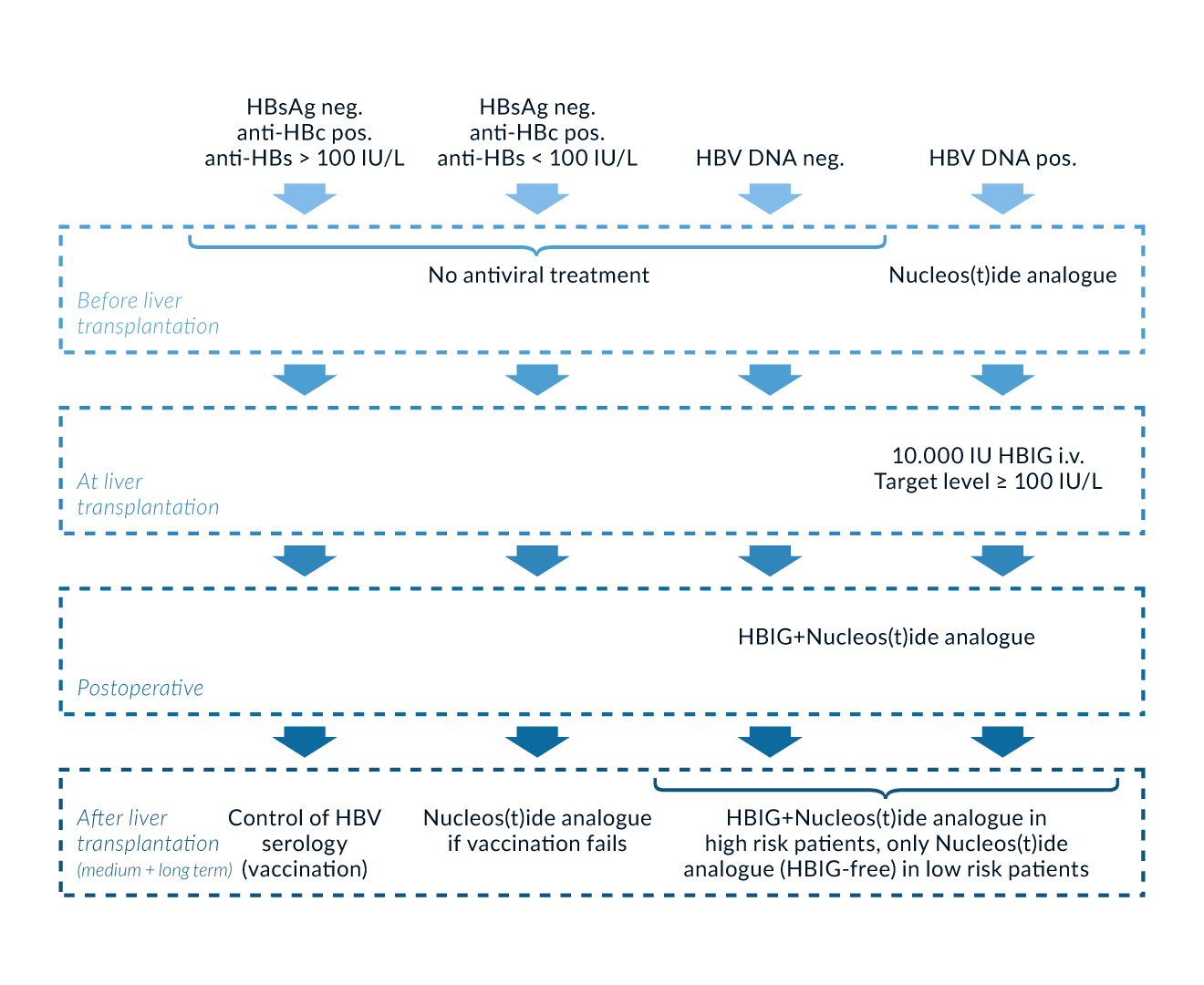 Figure 5. Prophylaxis of HBV recurrence after liver transplantation (LT). Postoperative combined use of nucleos(t)ide analog(s) and hepatitis B immunoglobulin (HBIG) is still the gold standard for prophylaxis of HBV reinfection early after LT. HBIG therapy can be withdrawn in the medium and long term after LT in low-risk patients. Those who are anti-hepatitis B core (anti-HBc) positive and without detectable anti-hepatitis B surface (anti-HBs) titres or with anti-HBs titres <100 IU/L should be vaccinated. In case of no or little response (anti-HBs <100 IU/L) to vaccination, lamivudine (LAM) monotherapy can be initiated. In patients who have protective anti-HBs titres of >100 IU/L, antiviral therapy is not necessary but long-term monitoring of HBV serology including anti-HBs titres is required. Neg., negative; pos., positive
Figure 5. Prophylaxis of HBV recurrence after liver transplantation (LT). Postoperative combined use of nucleos(t)ide analog(s) and hepatitis B immunoglobulin (HBIG) is still the gold standard for prophylaxis of HBV reinfection early after LT. HBIG therapy can be withdrawn in the medium and long term after LT in low-risk patients. Those who are anti-hepatitis B core (anti-HBc) positive and without detectable anti-hepatitis B surface (anti-HBs) titres or with anti-HBs titres <100 IU/L should be vaccinated. In case of no or little response (anti-HBs <100 IU/L) to vaccination, lamivudine (LAM) monotherapy can be initiated. In patients who have protective anti-HBs titres of >100 IU/L, antiviral therapy is not necessary but long-term monitoring of HBV serology including anti-HBs titres is required. Neg., negative; pos., positive
Monotherapy with entecavir or tenofovir in HBIG-free prophylactic regime have shown promising outcome in preventing HBV recurrence after LT (Orfanidou 2021). The efficacy of a switch after at least 12 months of HBIG/LAM to combination therapy with an oral nucleoside and nucleotide analogue was investigated (Saab 2011). Estimated HBV reinfection rate was 1.7% at 1 year after HBIG withdrawal.
A prospective, multicentre study in which 20 HBV patients received 800 IU HBIG (IM) in the anhepatic phase and for another 7 days after transplant surgery was published (Gane 2013). Patients with genotypic detection of LAM resistance and creatinine levels ≥ 1.8 mg/dL were excluded. ADV was administered as add-on therapy to existing LAM treatment. Previously untreated patients received combined ADV plus LAM treatment, which was continued after transplantation. Serum HBsAg and anti-Hbs were measured monthly in the first 3 months, then every 3 months. HBV DNA determination was only performed annually and at the end of the follow-up observation period. HBV recurrence was defined as the reappearance of HBsAg or detection of HBV DNA. The median follow-up was 57 months (range 27–83 months). At transplantation 68% of patients had demonstrable virus replication and 26% had viral replication >4 log10 IU/mL. After the end of the study, another 28 HBV patients received a liver allograft. The patients (n=18) who had HBV DNA <3 log10 IU/mL at transplantation were given no posttransplant HBIG therapy at all. The median follow-up was 22 months (range 10-58 months). Looking at both cohorts it was shown that there was a loss of HBsAg in 47/48 patients within 8 weeks posttransplantation and in one patient within 6 months after transplantation. In one patient with recurrence of HCC, there was a transient reappearance of HBsAg in the follow-up period.
In a randomised, prospective, controlled phase 2 trial, patients (n=40) received emtricitabine, TDF and HBIG for 24 weeks (Teperman 2013). Subsequently all patients who were negative for HBsAg and HBV DNA (<400 copies/mL) were randomly allocated to continue with all three drugs or to an arm with emtricitabine and TDF but without HBIG. The median period of time from LT was 3.4 years (range 1.9–5.6 years). During an observation period of 72 weeks, no HBV recurrence in terms of HBsAg or HBV DNA detection was observed in any of the patients.
Most HBV prophylactic posttransplant studies to date are limited, small and with short follow-up periods. EASL Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection (2017) recommend combined hepatitis B immunoglobulin (HBIG) and NUC for prevention of recurrent HBV infection after LT.
As a life-long therapy, this accounts in particular for patients with a high risk for HBV recurrence (HBV DNA positive at the time of LT, HBeAg positive, HBV underlying HCC, and HDV or HIV coinfection). A study by Saidy et al investigates the discontinuation of HBIG in patients after LT for combined HBV and HDV infection (Saidy 2021). In this small study 17 patients discontinued HBIG for various reasons. Graft function, overall survival and histopathological findings from routine liver biopsies were compared. No significant differences were found regarding the clinical course, histopathological findings or graft and patient survival. The authors assume that the duration of HBIG administration must be questioned. Other studies confirm that HBIG-free prophylaxis is not associated with a worse outcome (Dobrindt 2020). Suitable for this the EASL Clinical Practice Guidelines determine that patients with a low risk of recurrence can discontinue HBIG and proceed with indefinite nucleos(t)ide analogue monoprophylaxis.
According to updated AASLD Hepatitis B Guidance (Terrault 2018) prophylaxis with or without HBIG for 5-7 days and NUCs posttransplant followed by long-term potent NUC therapy in low-risk patients is an appropriate approach. ETV or TDF, an ester prodrug of tenofovir (TFV) or TAF, a phosphonate prodrug of TFV, with more favourable renal and bone safety than TDF are preferred antiviral drugs because of their low rate of resistance with long-term use. Combination antiviral therapy and HBIG is recommended by Terrault et al. (2018) for those with high risk of recurrent disease posttransplant (HDV- and HIV-coinfected patients and nonadherent patients).
For HBsAg negative LT recipients receiving HBsAg negative, anti-HBc– positive allografts, the reported risk of HBV transmission varies with the HBV immune status of the recipient. Those who have detectable anti-HBs titres have a significant lower risk as compared to those without detectable anti-HBc or anti-HBs titre. EASL Clinical Practice HBV Guidelines (2017) recommend LAM as prophylactic approach; whereas AASLD Hepatitis B Guidance (Terrault 2018) positively emphasises highly potent ETV, TDF or TAF for long-term prophylactic use in this scenario.
There is no rationale for continuing HBIG therapy in case of viral breakthrough with detectable HBV DNA. The choice of antiviral therapy in patients with HBV recurrence depends on the current antiviral medication, the viral load, and the resistance profile. Antiviral drug resistance can easily be established by genotypic assays that identify specific mutations known to be associated with decreased susceptibility to particular drugs.
Recurrence of hepatitis C in the allograft
HCV infection always recurs in the allograft in patients with detectable serum HCV RNA and according to EALS Practice Guidelines every recurrence should be treated (EASL 2020). The severity of HCV reinfection can be determined by liver biopsy. Transient elastography (TE) and acoustic radiation force impulse (ARFI) play a substantial complementary role for measurement of fibrosis in HCV and non-HCV transplant recipients (Cross 2011, Beckebaum 2010).
Antiviral treatment initiated after LT may be favourable after postoperative convalescence (approximately 3 months after LT). Patients with elevated liver enzymes and hepatic inflammation, portal hypertension, and/or the risk of rapid fibrosis progression should be treated earlier. Moreover, fibrosing cholestatic hepatitis (FCH) represents an urgent treatment indication. Studies based on smaller patient cohorts demonstrated excellent results in patients with FCH treated with sofosbuvir/Ledipasvir and ribavirin for 12 or 24 weeks (Charlton (a) 2015, Manns 2016). Treatment of severe recurrence after primary LT may therefore reduce the need for re-transplantation. Re-transplantation should be mentioned in acute liver failure after LT due to HCV-reinfection.
According to EASL Recommendations on Treatment of Hepatitis C (EASL 2020) patients with posttransplant HCV recurrence with non-cirrhotic changes of the allograft or with compensated cirrhosis (Child-Pugh A) should be treated with either: fixed-dose combination of sofosbuvir and velpatasvir for 12 weeks (no need for immunosuppressive drug adjustment) or fixed-dose combination of glecaprevir and pibrentasvir for 12 weeks (need for monitoring of drug levels and maybe adjustment of immunosuppressive medication). In patients with decompensated cirrhosis and recurrence of HCV fixed-dose combination of sofosbuvir and velpatasvir with daily weight-based ribavirin should be used for 12 weeks. In case of contraindications for ribavirin or poor tolerance to ribavirin on treatment sofosbuvir and velpatasvir should be used for 24 weeks without ribavirin.
The high level of safety and efficacy of direct-acting antiviral agents for HCV-treatment opens the opportunity to transplant organs from HCV positive patients into non-HCV positive patients, because these organs usually come from younger donors (Levitsky (b) 2017). HCV negative patients who receive an HCV positive organ should be treated in any case.
Recurrence of cholestatic liver disease and autoimmune hepatitis
Data on the frequency of recurrent cholestatic and AIH-related liver disease vary in the literature depending on the follow-up period and criteria chosen for definition of disease recurrence which may be more aggressive than the original disease in some transplant patients (Carbone 2014). The posttransplant prognosis for PBC patients is excellent, with an approximately 80% 5-year survival reported by most large centres (Carbone 2011, Silveira 2010). It has been reported that HLA-A, -B, and -DR mismatches between the donor and the recipient decrease the risk of disease recurrence in PBC patients (Morioka 2007a, Hashimoto 2001). A published study with long term follow-up data reported recurrent PBC in one-third of patients at 11-13 years posttransplant (Charatcharoenwitthaya 2007).
Diagnosis of PBC in the transplanted liver is usually more challenging than diagnosis in the native liver. Anti-mitochondrial antibodies (AMA) often persist, and elevated cholestatic enzymes may be due to other causes of bile duct damage such as ischemic cholangiopathy or chronic ductopenic rejection. Recurrent PBC is a histological diagnosis, typically appearing as granulomatous cholangitis or duct lesions. The frequency of recurrence will be considerably underestimated if a liver biopsy is carried out only when clinical features are apparent.
In a Japanese multicentre study, recipient aged 61 years or older, HLA mismatches of four or more (maximum of six), graft: recipient weight ratio less than 0.8, and husband donor were reported as negative predictors of patient survival in PBC patients after LDLT (Egawa 2016). Some investigators have found that CSA-based immunosuppressive therapy is associated with lower PBC recurrence rates as compared to TAC-based immunosuppression (Wong 1993, Montano-Loza 2010). However, long-term survival has been shown to be not significantly different between CSA- and TAC-treated patients (Silveira 2010). Recent data show that younger age at the time of PBC diagnosis or at LT, TAC use, and biochemical markers of cholestasis after LT are risk factors for PBC recurrence by the Global PBC Study Group (Montana-Loza 2019).
In the Mayo Clinic transplant cohort, 50% of recurrent PBC patients receiving UDCA showed normalisation of serum alkaline phosphatase and alanine aminotransferase levels over a 36-month period compared to 22% of untreated patients (Charatcharoenwitthaya 2007). Although no significant differences in the rate of histological progression was detected between the treated and untreated subgroups, the proportion of individuals with histological progression was significantly lower in those that showed improvement of biochemical parameters regardless of treatment.
A recently published multicentre study by Corpechot et al points out that preemptive therapy with UDCA is associated with reduced risk of disease recurrence, graft loss and liver- related and all-cause mortality (Corpechot 2020).
German Guidelines for autoimmune related liver diseases recommend use of UDCA in patients with recurrent PBC (Strassburg 2017). EASL Clinical Practise Guidelines on Liver Transplantation (2016) do not recommend so far prophylactic use of UDCA in patients transplanted for PBC and PSC (https://dx.doi.org/10.1016/j. jhep.2015.10.006).
Obeticholic acid (OCA) is a promising new therapy that has been shown to substantially improve the long-term outcomes of PBC patients with inadequate response or intolerance to UDCA in the non-transplant setting. However, data are awaited to examine the effects of OCA on clinical outcome in patients with recurrent PBC and the need for an alternative treatment option other than UDCA. Since bile salts are responsible for the secondary toxic consequences, bile salt and nuclear hormone directed therapies may improve secondary toxic injury and are under current investigation. However, so far, these drugs are not available yet.
The reported recurrence rates for PSC after LT range between 9% and 37% (Cholongitas 2008, Alabraba 2009, Vera 2002, Graziadei 1999, Goss 1997). Biliary complications and diagnosis of recurrent PSC can be easily managed in patients with duct-to-duct biliary reconstruction. While Roux-en-Y hepaticojejunostomy was previously the common anastomotic technique for LT in patients with PSC, duct-to-duct reconstruction is currently recommended if there is no evidence of pathological changes of the common bile duct.
German Guidelines for Autoimmune Related Liver Diseases state that UDCA can be used for patients transplanted for PSC as randomised controlled studies on the efficacy of UDCA in patients transplanted for PSC are not available (Strassburg 2017). UDCA does not seem to have an influence on PSC recurrence rates. Preclinical studies in the non-transplant setting suggest that FXR- and PPAR-agonists, inhibitors of the apical sodium-dependent bile salt transporter (ASBT-inhibitors) and the C23 UDCA derivative nor-UDCA are promising agents for the treatment of PSC. However, data from studies targeting new therapeutic approaches in LT patients with recurrent PSC are not available.
In patients who underwent LT for PSC tacrolimus is associated with a better patient and graft survival compared to cyclosporine, tacrolimus should be the standard calcineurin inhibitor in those patients (Aberg, 2024).
Various risk factors for PSC recurrence have been identified including the presence of cholangiocarcinoma prior to LT; presence of certain human leukocyte antigen (HLA) such as HLA-DRB1*08, HLA DR52 in the recipient or donor; male recipient, a recipient-donor gender mismatch; recipient age, an intact colon in the recipient prior to LT, the presence of ulcerative colitis and early cholestasis after LT; use of extended donor criteria grafts; acute cellular rejection, steroid-resistant acute cellular rejection or use of OKT3; maintenance of steroid therapy for ulcerative colitis for more than 3 months; and CMV infection in the recipient (Faisal 2015, Montano-Loza 2016 ). An increased risk of recurrence has been reported in recipients of grafts from first-degree living related donors in two small single centre series from Japan (Tamura 2007, Haga 2007). A recently published study by Visseren et al detected specific difference in the gut microbiome pre transplantant in patients with recurrence of PSC and those without after LT (Visseren 2020). No difference in the alpha- or beta diversity were observed between recurrence and no-recurrence, but many over-represented bacterial features were detected in patients with recurrence of PSC. Further investigation in bacterial difference in needed.
Recurrent PSC is diagnosed by histology and/or imaging of the biliary tree and exclusion of other causes of non-anastomotic biliary strictures. Histopathological findings in PSC include fibrous cholangitis, fibroobliterative lesions, ductopenia, and biliary fibrosis.
It has been described that recurrence of PBC and AIH does not significantly impact long term outcome including overall survival whereas recurrent PSC has been associated with a higher re-transplantation rate (Tanaka 2020).
A British LT group found significantly better recurrence-free survival rates in patients who underwent colectomy before or during LT and in those with with non-extended donor criteria allografts (Alabraba 2009).
Interestingly, despite immunosuppression, a significantly higher corticosteroid requirement was reported in the transplant compared to the non-transplant setting, with 20% of PSC patients with concomitant PSC becoming corticosteroid dependent after LT (Ho 2005). A recent study reported that maintenance steroids (>3 months) for ulcerative colitis post-LT were a risk factor for recurrent PSC (Cholongitas 2008). A Scandinavian group studied the risk of colorectal neoplasia among 439 PSC patients, 80% of whom had chronic inflammatory bowel disease prior to LT and 3% of whom had developed de novo chronic inflammatory bowel disease (Jørgensen 2012). The median history of chronic inflammatory bowel disease was 15 years (range 0–50 years) and the follow-up period posttransplantation was 5 years (range 0–20 years). A fourth of the PSC patients who additionally had bowel involvement developed colorectal neoplasias. This frequency was twice as high postoperatively than before LT. Patients receiving TAC and MMF had a significantly higher risk of chronic inflammatory bowel disease-associated active inflammation than patients taking CSA and azathioprine (Jørgensen 2013). Morover, a Swedish study (Lindström 2018) TAC was reported as an independent risk factor for PSC recurrence. However, due to conflicting results in literature, impact of immunosuppression on PSC recurrence needs further investigation.
AIH recurrence was 20% after 5 and 31% after 10 years respectively in a recently published multicentre study (Montano-Loza 2022). Recurrence of AIH was associated with younger age at transplantation, immunosuppressive therapy with mycophenolate mofetil, sex mismatch and high immunoglobuline G before LT. Recurrence of AIH is a risk factor for impaired graft function and overall survival.
Transplantation centres commonly maintain AIH patients on prednisone after LT to reduce rejection and recurrence rates. However, there is limited evidence for this approach (Stirnimann 2019) and impact of type and dosing of immunosuppressive drugs on outcome needs further investigation. Survival rates post-LT are approximately 90% and 70% at 1 and 5 years (Montano-Loza 2016). A long-term follow-up study (>10 years) by a French group found AIH recurrence in 41% of the patients. The authors recommended regular liver biopsies, because histological signs precede abnormal biochemical liver values in about one-fourth of patients (Duclos-Vallee 2003). The diagnosis of recurrent AIH may include histological features, the presence of autoantibodies, and increased gamma globulins. Histological signs of recurrence include interface hepatitis, lymphoplasmacytic infiltration, and/or lobular involvement. The majority of published studies did not confirm a posttransplant prognostic role of antibodies in patients undergoing LT for AIH. Conflicting data exist regarding the presence of specific HLA antigens that predispose patients to AIH recurrence after LT (Gonzalez-Koch 2001, Molmenti 2002).
Recurrent AIH must be distinguished from de novo AIH, which is a clinical entity resembling AIH and develops in LT recipients transplanted for other liver disorders. It was originally described in children after LT. The incidence of de novo AIH is variable because multiple descriptions have been used in case series. The Banff working group on liver allograft pathology has recently suggested that the nomenclature ‘de novo AIH’ should be replaced by the terminology ‘plasma-cell rich rejection’ (Montano Loza 2016, Demetris 2016).
Outcome and recurrence in patients transplanted for hepatic malignancies
The results of early studies of LT for HCC were disappointing. More than 60% of patients developed tumour recurrence within the first two years posttransplant (Ringe 1989). Currently, there are recurrence rates of 10-15% in patients fulfilling the Milan criteria (Zavaglia 2005) and the majority of recurrence occurs within the first two years after LT (Stras 2022). A recurrence after five years is rare. In analyses of predictors of survival histological grade of differentiation, macroscopic vascular invasion and satellitosis were identified as independent predictors of survival and tumour recurrence (Zavaglia 2005, Hoyos 2015). Others identified MELD score >22, AFP >400 ng/mL and age >60 years as negative predictors for survival in HCC (Sotiropoulos 2008b, Jelic 2010). Several retrospective cohort studies are published in literature which demonstrated statistically significant differences in survival and recurrence between different RECIST criteria after LT (Morris 2016). AFP independently predicts tumour recurrence and correlates with vascular invasion and differentiation (Duvoux 2016). A French group of researchers developed a selection model called the AFP score. This score allows patients with HCC not meeting Milan criteria but scored 2 or lower, with AFP levels less than 100 ng/mL and a low 5-year risk of recurrence to be transplanted with excellent results (Duvoux 2016). In another study, Notarpaolo (2016) tested this AFP score in a population of non-French patients transplanted for viral hepatitis underlying HCC. The authors concluded that in this specific population, the AFP model better selects patients with HCC as compared to Milan criteria and that the AFP score can also be implemented in countries with an important burden of HCC occurring on post-hepatitic cirrhosis.
For patients having an indication for LT despite exceeding the Milan criteria, the use of marginal grafts or performance of LDLT has been considered as a reasonable option.
Expansion beyond the Milan criteria to University of California San Francisco (UCSF) criteria (single tumour <6.5 cm; two to three tumours, none >4.5 cm or total diameter <8 cm, no vascular invasion) or even more liberal criteria (no portal invasion, no extrahepatic disease) have been discussed widely (Sotiropoulos 2007, Silva 2011, Jelic 2010). Centres such as the San Francisco Transplant Group as well as the UCLA Transplant Group have demonstrated 5-year survival rates of 50-80% after LT for tumours beyond the Milan criteria but within UCSF criteria (Duffy 2007, Yao 2007). In a recently published study (Victor 2020) from the Houston transplant group, 220 HCC patients were transplanted, 138 inside Milan, 23 inside UCSF, and 59 beyond UCSF criteria. Interestingly, patient survival was similar at 1, 3, or 5 years despite pathologic tumour size.
The ‘up to seven’ criteria (7 being the sum of the size and number of tumours for any given HCC) was suggested as an approach to include additional HCC patients as transplant candidates. However, acceptance of a more liberal organ allocation policy would result in a further increase of HCC patients on the waiting list and in denying the use of these organs to other non-HCC patients.
The existence of several scoring systems in this era of LT shows on the one hand the widely held conviction of the transplant community that the well-established Milan criteria are too restrictive, not allowing many HCC patients the LT opportunity; on the other hand, this situation reflects some limitations of the existing pretransplant radiological evaluation (Sotiropoulos 2009). Multiple reports in the radiology literature address nodule detection in cirrhotic livers by means of CT, MRI, or ultrasonography. Many of them conclude that contrast-enhanced MRI is the most sensitive technique for detecting liver nodules (Teefey 2003, Tokunaga 2012). MRI has been shown to depict only 39 of 118 HCC in cirrhosis, for an overall sensitivity of 33% (Krinsky 2002). Detection of small tumours was inadequate, with only 11 of 21 lesions (52%) between 1 and 2 cm and 3 of 72 lesions (4%) <1 cm correctly classified. The sensitivity in the series from Essen was similarly poor, 0% for tumours <1 cm and 21% for tumours between 1 and 2 cm (Sotiropoulos 2005). Similar findings have been reported (Bhartia 2003) with the conclusion that the identification rate of tumours <1 cm is still limited. The presence of microvascular invasion and, in some cases, macrovascular invasion of segmental branches can usually be determined by pathologic inspection of the explanted liver. This, together with inaccurate tumour detection, leads to upgrading of the tumour stage or the classification according to the different sorts of criteria in the posttransplant period, compared to assumed stages by radiological evaluation. More important, however, is the fact that some patients might not be given the opportunity to undergo LT on the basis of inaccurate radiological and clinical preoperative staging.
Mazzaferro et al. (2018) found that patients with HCC achieve a 70% chance of HCC-specific survival 5 years after LT, if AFP level are <200 ng/ mL and the sum of number and tumour size (in centimeters) do not exceed 7. The authors created a model comprising level of AFP, tumour size, and tumour number, to determine the risk of death from HCC-related factors after LT and to define selection criteria for LT in HCC patients. For this purpose they provided an online calculator to predict 5-year survival and risk of HCC-related death.
Expansion of criteria in the LDLT setting is even more challenging due to the donor risk and the risk of selection of tumours with unfavourable biology following the concept of fast-tracking (Hiatt 2005). Novel molecular biology techniques, such as genotyping for HCC, may become relevant for determining recurrence-free survival and improving patient selection, but these biomarkers can not yet be used for clinical decision making.
A potential survival benefit was reported in studies and a meta-analysis of controlled clinical trials with SRL-based immunosuppression in patients transplanted for HCC (Kneteman 2004, Zimmerman 2008, Toso 2007, Liang 2012). These results are in line with a retrospective analysis based on the Scientific Registry of US Transplant Recipients, which included 2491 HCC LT recipients and 12, 167 recipients with non-HCC diagnoses. Moreover, the SILVER Study, a large prospective RCT, comparing SRL-containing versus SRL-free immunosuppression showed a benefit in recurrence-free survival and overall survival in the SRL group in the first 3 to 5 years, in particular in low risk patients, but did not improve long-term recurrence-free survival beyond 5 years (Geissler 2016).
Sorafenib (SOR) is currently used for HCC recurrence after LT when patients are not suitable for surgical/locoregional treatments. Repeated LT is not recommended (Stras 2022). In an Italian study (Invernizzi 2019) treatment response was obtained in 16% and stable disease in 50% in those treated with SOR (74% were on mTOR inhibitors). Median time to radiological progression was 6 months. Baseline predictors of overall survival were SOR+mTOR inhibitors, previous curative treatments and AFP>100 ng/mL. In addition Lenvatinib is used for recurrence treatment in some centres.
Although initial post-LT survival rates were poor in patients with unresectable hilar CCA outcomes, after introduction of the Mayo Clinic protocol, outcomes have been more promising. Neoadjuvant chemoradiation and subsequent LT has shown promising results for patients with localised, unresectable hilar cholangiocellular carcinoma (CCC) (Welling 2014, Masuoka 2011). In a published US study, the outcome of 38 patients who underwent LT was compared to that of 19 patients who underwent combined radical bile duct resection with partial hepatectomy (Hong 2011). The tumour was located in the intrahepatic bile duct in 37 patients and in the hilar bile duct in 20 patients. Results demonstrated that LT combined with neoadjuvant and adjuvant therapies is superior to partial hepatectomy with adjuvant therapy. Challenges of LT attributable to neoadjuvant therapy include tissue injury from radiation therapy and vascular complications including HAT. Predictors of response to the neoadjuvant protocol prior to LT need to be determined (Heimbach 2008). Increasing age, high pretransplant tumour marker, residual tumour size in the explant >2 cm, tumour grade, previous cholecystectomy and perineural invasion were identified as predictors of recurrence following LT (Knight 2007).
Machairas et al. (2020) conducted a systematic review investigating longterm outcomes of patients (n=698) with hilar CCC undergoing LT. A total of 13 studies were included in this systematic review. The majority (74.4%) received neoadjuvant therapy (combined chemotherapy and radiation). One-, 3- and 5-year overall survival rates ranged between 58%-92%, 31%80% and 20%-74%, respectively. Recurrence rates ranged widely between 16% and 61%, and perioperative mortality ranged between 0% and 25.5%. Results revealed that LT could provide acceptable long-term outcomes in the setting of neoadjuvant therapy using strict patient selection criteria.
Metastatic lesions originating from neuroendocrine tumours (NET) may be hormone-producing (peptide hormones or amines) or may present as nonfunctional tumours (Frilling 2006). They are characterised by slow growth and frequent metastasis to the liver, and their spread may be limited to the liver for protracted periods of time. Most studies in patients transplanted for NET are limited and usually restricted to small numbers of patients. An analysis based on the UNOS database including patients transplanted for NET between October 1988 and January 2008 showed that long-term survival of NET patients was similar to that of patients with HCC. Excellent results can be obtained in highly selected patients and a waiting time for LT longer than 2 months (Gedaly 2011). A recently published study with 32 patients showed excellent long-term survival rates even in patients with post-LT NET recurrence (particulary in late recurrence >24 month after LT) in particular by aggressive surgical treatment (Sposito 2021). Long-term results from prospective studies are needed to further define selection criteria for patients with NET for LT, to identify predictors for disease recurrence, and to determine the influence of the primary tumour site on patient posttransplant survival.
Recurrent alcohol abuse after liver transplantation for alcoholic liver disease
Recent trials have shown that uEtG or hair-EtG determinations are reliable markers for detection of alcohol relapse after LT (Staufer K 2011). Reported rates of returning to drinking after LT for ALD vary in the literature. Studies revealed a mean incidence of relapse in one-third of patients ranging from 10% to 50% in up to 5 years of follow-up (EASL CPG Management of alcohol-related liver disease [2018]). Approximately 10% to 15% of patients with recurrent ALD resume heavy drinking with damage of the new liver (Marroni 2018). There are psychological scoring systems to assess the relapse risk in patients with alcohol abuse but a prospective validation is missing (Shenoy 2021). Among other things the Sustained Alcohol Use Post-LT (SALT) score score by Lee et al was published (Lee (b) 2019). This prognostic score using four objective pretransplant variables (>10 drinks per day at initial hospitalisation, multiple prior rehabilitation attempts, prior alcohol-related legal issues and prior illicit substance abuse) identifies candidates with AH for early LT who are at low risk for sustained alcohol use posttransplant.
Marot et al. (2018) performed a systematic review and metaanalysis in patients with AH. Pooled estimated risk for alcohol relapse was 0.22. This risk was not statistically significant different between AH and AC with 6 months of abstinence. Pooled estimated rate for 6 month survival was 0.85 and similar between both groups.
Predictors of recurrence include positive family history of substance use, pretransplant abstinence, failed rehabilitation attempts, history of prior alcohol-related legal issues, history of substance abuse (other than alcohol), smoking, lack of social support, lack of familiar support, denial of drug-related problems and addiction, length and intensity of alcoholic liver disease and psychiatric comorbidities (Perney 2005, Dew 2008).
Patient and graft survival is excellent in those maintaining alcohol abstinence after LT. A study (Parrish 2019) considering SRTR data from patients (n=53.788) transplanted between 2014 and 2017 showed that patients with ALD and HCV had superior graft survival rates (90.7% at 1 year, 78.9% at 3 years and 90.0% at 1 year, 79% at 3 years, respectively) as compared to those with nonalcoholic steatohepatitis (NASH) (87.5% at 1 year, 77.9% at 3 years).
The American Consortium of Early Liver Transplantation for Alcoholic Hepatitis analysed outcome of early LT for patients without mandatory period of sobriety with severe alcoholic hepatitis. Data derived from 12 centres from 8 UNOS regions (Lee (c) 2018). The authors reported a cumulative incidence of any alcohol use (slips or sustained alcohol use) of 25% at 1 year (95% CI, 18%-34%) and of 34% at 3 years (95% CI, 25%-44%) after LT. The cumulative incidence of sustained alcohol use was 10% at 1 year (95% CI, 6%-18%) and 17% at 3 years (95% CI, 10%-27%) after LT. Patients overall survival after 1 year (94%) and 3 years (84%) was not significantly worse compared to patients undergoing LT for other indications but sustained drinking after LT was associated with increased mortality (hazard ratio, 4.59; P=.01). A significant decrease of the medium- and long-term survival in severe chronic alcohol consumption after LT has also been shown in previous studies (Pfitzmann 2007).
For LT recipients with a history of ALD (and positive smoking history), a more intensive surveillance protocol including annual skin and ear nose throat (ENT) examinations as well as upper endoscopy (every 2–3 years) and abdominal ultrasound should be considered. Modifiable factors such as life style habits including cigarette smoking, physical inactivity, and obesity should be avoided. A systemic evaluation including malnutrition, vitamin and trace element deficiency, and osteoporosis is recommended.
According to results from the European Liver Transplant Registry (ELTR), mortality and graft failure were more often related to de novo tumours, cardiovascular and social factors in alcoholic LT patients as compared to patients transplanted for other etiologies (Burra 2010). LT recipients with a prior diagnosis of ALD might benefit from immunosuppressive regimens that minimise CNI exposure and favour mTOR-containing regimes. However, prospective studies are needed to gain more insight into this issue.
Recurrent non-alcoholic fatty liver disease
The increasing incidence of obesity and the metabolic syndrome throughout developed countries results in an increasing proportion of patients transplanted for NAFLD (Darwid Murash 2015). Younossi et al. (2016) constructed a steady-state prevalence model to quantify the economic and clinical burden of NAFLD in the United States and Europe. Data were validated using a computerised disease model. In the United States, over 64 million people are projected to have NAFLD, with an annual direct medical burden of approximately $103 billion ($1, 613 per patient). In Germany, France, Italy, and United Kingdom, the authors estimated ~52 million people with NAFLD with an annual cost of approximately €35 billion (from €354 to €1, 163 per patient). Life style interventions are of utmost importance and overweight patients who achieve significant reductions in body weight through physical activity and low caloric diet can decrease liver fat, visceral and subcutaneous adipose tissue (Copaci 2015). Treatment of NAFLD will likely involve a holistic, multidisciplinary and personalised approach (Malhotra 2015).
Patients transplanted for NAFLD had similar outcomes compared with patients transplanted for other indications (Burra 2014). Reported NAFLD recurrence rates after LT vary in the literature, ranging between 20 and 40%. Villeret et al maintain that the recurrence of the underlying disease is inevitable and progressive in a large proportion of patients who underwent LT for NAFLD cirrhosis (Villeret 2023). This leads to a higher attention to life style changes after LT. The components of metabolic syndrome are often exacerbated following LT by factors such as immunosuppression requiring an aggressive management of cardiovascular complications after transplantation.
The transplant group from Stockholm (Tokodai 2019) conducting a retrospective study identified recipient age and 1-year BMI in multivariate analysis as independent risk factors for post-LT fatty liver disease development. Weight gain after LT is significantly greater in patients with older age (>50 years) and in those transplanted for chronic compared with fulminant liver failure. Thus, at least for steroid-free regimens, weight gain seems to be unrelated to any specific immunosuppressive drug. The greatest weight gain has been observed after the first 6 months posttransplant. Physical activity in LT recipients should be proposed as part of their therapeutic regimens. It also appears to improve health-related quality of life after LT (Battistella 2022), thus regular exercise programmes and a healthy diet may be incorporated to avoid cardiovascular morbidity and mortality and NAFLD recurrence (Cotter 2020).
There are continuous efforts on finding novel agents to help prevent and to slow down the progression of recurrent NAFLD (Younossi ZM 2019, Tang 2019). The importance of the gut microbiome in mediating hepatocyte inflammation and intestinal permeability may also offer future treatment options.
Pregnancy after liver transplantation
Adequate preconception counseling is crucial to provide optimal conditions for pregnancy and to modify immunosuppressive therapy if necessary to minimise risks for both the mother and the fetus. Female LT patients of reproductive age should preferentially use contraception during the first 12 months after transplantation. Immunosuppression therapy should be continuied during pregnancy, however, individual regimens could be possible (Rahim 2020). Fetal loss, prematurity, and low birth weight have been reported in women who have undergone transplantation (Valentin 2021), and maternal risks include hypertension, preeclampsia, gestational diabetes, and graft dysfunction. The rate of caesarean section is considerably higher in post-LT patients. Steroids, CNIs have not been reported to be teratogenic and should be maintained during pregnancy; whereas mycophenolate mofetil has shown to cause malformations in animal models and should be avoided. mTOR inhibitors may affect spermatogenesis in male recipients. More studies should be designed to investigate the role of immunosuppression on sexual dysfunction. In a retrospective study by Zaffar et al. (2018) 41 pregnancies in 28 transplanted women were considered. Mean transplant-to-pregnancy interval was 8.5±5.1 years. Immunosuppressive therapy consisted of TAC ± azathioprine (n=26), CSA (n=4) and prednisone with other immunosuppressive drugs (n=11). During pregnancy the following adverse events have been reported: hypertension (n=10), impairment of renal function (n=6), gestational diabetes (n=4), impairment of allograft function (n=2), and blood transfusion requiring anaemia (n=1). Two miscarriages, three stillbirths and one neonatal death occurred. Moreover, five small-for-gestational-age infants, one minor congenital anomaly and premature delivery in fourteen infants (38.9%) have been reported.
Although there is an increased risk for pregnancy-related complications as compared to the general population an appropriate multidisciplinary care, stable graft function at pregnancy onset and adherence to immunosuppressive regimens are a good prerequisite for a successful pregnancy and delivery after LT.
Experiences with liver transplantation in inherited metabolic liver diseases in adult patients
LT is regarded as an effective treatment strategy for patients with Wilson’s Disease, which presents as deterioration of cirrhosis not responsive to treatment, as acute-on-chronic disease or fulminant hepatic failure (Moini 2010). LT reverses the abnormalities of copper metabolism by converting the copper kinetics from a homozygous to a heterozygous phenotype, thus providing an adequate increase of ceruloplasmin levels and a decrease of urinary copper excretion posttransplant. 1- and 5-year survival is excellent with 88% and 83% respectively (Ferrarese 2020). There are several reports in the literature indicating a reversal of neurological symptoms after LT (Martin 2008, Poujois 2020). However, the course of neurological symptoms remains unpredictable and it is still a matter of debate whether LT should be considered in patients with severe neurological impairment (Pabón 2008).
AAT deficiency is a common genetic reason for paediatric LT, but a rare indication in adults. The Z allele is most commonly responsible for severe deficiency and disease. LT corrects the liver disease and provides complete replacement of serum AAT activity. 567 AAT recipients who underwent LT between 1995 and 2004 were retrospectively investigated (Kemmer 2008). Survival rates after LT for AAT are excellent (1-year 93%, 5-year 90%, 20-year 82%) (Guillaud 2021).
In haemochromatosis, iron depletion therapy prior to LT may be associated with a better outcome after LT and is strongly recommended (Weiss 2007). It has been reported that the survival of patients who undergo LT for hereditary haemochromatosis is markedly lower in comparison to other indications (Dar 2009, Brandhagen 2001). Reduced posttransplant survival in patients with haemochromatosis has been attributed to cardiac problems and increased infectious complications. Findings derived from the UNOS database revealed 1- and 5-year survival rates of 75% and 64% in patients with iron overload, as compared to 83% and 70% in those without iron overload (Brandhagen 2001). More recent results from patients with haemochromatosis (n=217) transplanted between 1997 and 2006 revealed excellent 1- (86.1%), 3- (80.8%), and 5-year (77.3%) patient survival rates, which were not different from those transplanted for other liver diseases (Yu 2007).
LT halts production of mutated transthyretin (TTR) and therefore represents an accepted treatment for hereditary transthyretin (ATTR) amyloidosis, a systemic amyloidosis mainly affecting the peripheral nervous system and heart (Rocha 2016). Okumura et al. (2016) recently assessed 29 non-transplant and 36 transplant FAP V30M patients using an FAP clinical scoring system. They found that LT had beneficial effects on FAP clinical manifestations in these patients. However, the effects of transplantation on the clinical manifestations of FAP have not been systematically investigated and future studies are urgently warranted.
Outcome after liver transplantation for acute and acute-on-chronic liver failure
About half of acute hepatic failure (AHF) patients undergo LT. ALF accounts for 5-12% of LT activity worldwide and 7.3% in Europe (http://www.eltr.org/Overall-indication-and-results.html)
Of patients listed for transplantation, approximately one third will recover spontaneously without the need for grafting; thus, in as many as 20% of ALF patients LT is required (Lee 2012). Transplantation should be considered in those patients fulfilling Clichy or Kings College criteria (EASL CCPG on the Management of Acute (Fulminant) Liver Failure (2017); http://www.easl.eu/medias/cpg/ALF/English-report.pdf). Drug-induced liver injury due to acetaminophen overdose is the most common cause of LT for acute liver failure in developed countries (Craig 2010, Au 2011). Other etiologies comprise idiosyncratic drugs (such as isoniazid/rifampicin, cumarins, acetaminophen, ectasy, tricyclic antidepressants), Budd-Chiari syndrome, Wilson’s Disease, hepatitis A, B and E infection or autoimmune disease.
Early postoperative complications in patients transplanted for AHF include sepsis, multisystem organ failure, and primary graft failure. Serum creatinine concentrations above 200 µmol/L pretransplant, non-white race of the recipient, donor body mass index >35 kg/m2 and recipient age >50 years have been suggested as risk factors for posttransplant mortality (Wigg 2005). Others reported that extended donor criteria rates and severe cerebral edema were associated with worse outcome (Chan 2009). The Edinburgh LT centre investigated the impact of perioperative renal dysfunction on posttransplant renal outcomes in AHF patients. They found that older age, female gender, hypertension, CSA and non-acetaminophen-induced AHF but not the severity of perioperative renal injury were predictive for the development of chronic kidney injury (Leithead 2011).
The results in patients transplanted for AHF have improved within the last decade due to the establishment of prognostic models, improved intensive care management and the option for LDLT which has a limited role in the US and Europe but plays a major role in Asia (Lo 2008). AHF was the indication for LDLT in more than 10% of the cohort reported by two Asian groups (Morioka 2007b, Lo 2004).
It has been reported that survival in patients with AHF is inferior to that of recipients with non-acute indications for LT in the first year but comparable in the long-term (Chan 2009, Wigg 2005). The US Acute Liver Failure Study Group found that two-year outcomes in initial survivors of AHF are generally good but that non-acetaminophen patients have a significantly lower survival, which may be related to pre-existing medical comorbidities (Fontana 2015).
Acute-on-chronic liver failure (ACLF) is characterised by acute decompensation of liver cirrhosis and is often combined with severe systemic inflammation, organ failure and a high mortality (transplantation-free-28-day mortality of 33%) (Schulz 2022). 1- year survival rates after LT for ACLF range from 70 to 80% depending on patient population and ALF severity. In recently published studies survival do not differ significantly from patients without ACLF (Schulz 2022). Further studies will be needed to improve current transplant allocation system for patients with this severe syndrome.
Conclusion
- LT is often the only life saving therapy in patients with acute liver disease, chronic liver disease or HCC
- Alcoholic and viral hepatitis are the most common reasons for LT worldwide, NAFLD is a strongly increasing
- The allocation system using the MELD score (creatinine, bilirubine and INR) optimises the priority of patients with severe liver disease
- Hypothermic machine perfusion expands the pool of usable livers
- Lifelong surveillance after LT is necessary to detect immunosuppression side-effect, graft failure or recurrence of underlying disease after LT
- Tailored immunsuppressive regims are necessary to improve graft and patient survival
LT is challenging due to a shortage of organs and a prolonged waiting-list time. The large disparity between the number of available deceased donor organs and recipients awaiting LT has created an ongoing debate regarding the appropriate selection criteria. A variety of approaches have been implemented to expand the organ donor pool including national efforts to expand deceased donor donation, split organ donations including LDLT, increased use of more elderly and obese donors and greater utilisation of expanded criteria donors. The rationale of allocation systems utilising the MELD score is to prioritise patients with severe liver dysfunction (“the sickest first”). This results in decreased waiting list mortality from 20 to 10% in the Eurotransplant region but also in a reduction of 1-year posttransplant survival by approximately 10%. A potential modification of the MELD allocation system or development of an improved prognostic scoring system is urgently warranted to optimise organ allocation in the future and to adjust gender difference.
Due to the availability of antiviral drugs, the survival of patients undergoing LT for HBV infection has dramatically improved and has become comparable to or even better than the survival of patients with nonvirus-related liver diseases. Protocols have been published in literature implementing withdrawal of HBIG or HBIG-free regimens, using only oral antivirals, in particular in patients at low risk of recurrence.
The availability of DAA all-oral combinations constitutes a substantial improvement in HCV therapy and in particular in patients formerly difficult-to-treat such as cirrhotic patients and in managing HCV infection after LT. SVR rates in LT patients are comparable with nontransplant patients and can be achieved with excellent tolerability.
Expansion of the donor pool by including HCV positive organs in the DAA era could substantially decrease waiting times and mortality rates for patients listed for LT. Mounting data demonstrate the safety of using organs from HCV-infected donors with subsequent treatment of HCV in the recipient. However, use of HCV positive donors in HCV negative LT recipients may currently be restricted to urgent situations and necessitates a robust informed consent process.
Data about the frequency of disease recurrence in cholestatic and autoimmune liver diseases vary in the literature. Diagnosis of disease relapse in cholestatic and autoimmune liver disease is more challenging than in the non-transplant setting. Most studies report excellent mediumterm and long-term results despite limited therapeutic options for disease recurrence.
LT in HCC patients provides excellent outcomes and low recurrence rates following the Milan criteria. Expansion of transplantation criteria beyond the Milan criteria has been discussed at length. The acceptance of a more liberal organ allocation policy may result in a further increase of the proportion of patients transplanted for HCC and denying the use of these organs to other patients for whom better results may be achieved. Recent developments in genomic and proteomic approaches may allow the identification of new biomarkers for prediction of HCC recurrence.
ALD is the leading indication for LT in European and US transplant centres. Early LT without fulfilling the 6-month abstinence rule should be restricted to those with severe disease who are not responding to medical therapy, have been subjected to a careful selection process and have a favourable addiction and psychosocial profile. German regulations require 6 months of alcohol sobriety in patients with ALD, however, in exceptional cases patients can get access to the waitlist through an audit process requested by the corresponding transplant centre and organised by Eurotransplant.
There should be psychosocial evaluation of the patient with ALD prior to LT considering possible risk factors for recurrence. Implementation of prognostic instruments for prediction of alcohol relapse are recommended. ALD patients on the waiting list should be monitored for alcohol use by regular clinical interviews and laboratory tests to confirm abstinence. However, standardisation and unified policy of the selection process may be helpful. Prospective studies are urgently needed to resolve the controversies that still surround the criteria for selection of those patients for LT.
The management of cardiovascular, renal, coagulopathic, cerebral and infectious complications in patients with AHF is clinically challenging. Prognostic models are helpful but not entirely accurate in predicting those who will require LT. Due to advances in intensive care medicine and surgical techniques, outcomes for patients with AHF have progressively improved over the last 2 decades.
CNI, at least at low doses, with or without other immunosuppressive drugs, have been so far the cornerstone of immunosuppressive regimens in a substantial proportion of LT patients. Much attention has been directed to reducing CNI-associated long-term complications. Cardiovascular comorbidities due to metabolic complications such as diabetes mellitus, dyslipidaemia, obesity, and arterial hypertension account for 30-70% of long-term morbidity. Current trends of immunosuppressive strategies include CNI-sparing or CNI-free protocols including MMF- and/or mTOR-based immunosuppressive regimens and corticosteroid-avoidance protocols. mTOR-based immunosuppression should be used in HCC-patients due to antitumour effects. CNI delay with induction therapy for bridging the early postoperative phase should be considered especially in patients with high MELD scores. Finally, “individually tailored immunosuppressive” protocols may optimise drug efficacy, minimise drug toxicity and improve transplant outcome.
References
AASLD/IDSA HCV Guidance Panel. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology 2015;62(3):932-54. doi: 10.1002/hep.27950.
Abbas Z, Afzal R. Hepatitis E: when to treat and how to treat. Antivir Ther 2014;19(2):125-31.
Addolorato G, Bataller R, Burra P et al. Liver Transplantation for alcoholic liver disease. Transplantation 2016;100:981-98. doi:10.1097/, TP.0000000000001156.
Åberg F, Sallinen V, Tuominen S, European Liver and Intestine Transplant Association (ELITA). Cyclosporine vs. tacrolimus after liver transplantation for primary sclerosing cholangitis - a propensity score-matched intention-to-treat analysis. J Hepatol. 2024 Jan;80(1):99-108.
Alabraba E, Nightingale P, Gunson B, et al. A re-evaluation of the risk factors for the recurrence of primary sclerosing cholangitis in liver allografts. Liver Transpl 2009;15:330-40.
Alim A, Erdogan Y, Dayangac M, et al. Living Donor Liver Transplantation: The Optimal Curative Treatment for Hepatocellular Carcinoma Even Beyond Milan Criteria. Cancer Control. 2021.
Angus PW, Strasser SI, Patterson S, et al. A randomised study to assess the safety and efficacy of adefovir dipivoxil substitution for hepatitis B immune globulin in liver transplantation patients receiving long-term low dose IM HBIG and lamivudine prophylaxis. Hepatology 2007;46:238A.
ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65(4):734-40.
Au JS, Navarro VJ, Rossi S. Drug-induced liver injury – its pathophysiology and evolving diagnostic tools. Aliment Pharmacol Ther 2011;34:11-20.
Avery RK, Alain S, Alexander BD; SOLSTICE Trial Investigators. Maribavir for Refractory Cytomegalovirus Infections With or Without Resistance Post-Transplant: Results From a Phase 3 Randomised Clinical Trial. Clin Infect Dis. 2022 Sep 10;75(4):690-701.
Bajjoka I, Hsaiky L, Brown K, Abouljoud M. Preserving renal function in liver transplant recipients with rabbit anti-thymocyte globulin and delayed initiation of calcineurin inhibitors. Liver Transpl 2008;14:66-72.
Baker TB. Living liver donation, donor safety, and social media: Preparing for a new frontier. Liver Transpl 2017;23(2):131-132.
Barosa R, Teixeira C, Pinto J, E Branco JC. Early liver transplantation for severe alcoholic hepatitis - are we exploring all the tools? Liver Int 2017;37(9):1407-8.
Battistella S, D'Arcangelo F, Grasso M, et al. Liver Transplantation for NAFLD: indications and posttransplant management. Clin Mol Hepatol. 2022.
Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients. Liver Transpl 2002;8:110-7.
Beckebaum S (a), Cicinnati V, Brokalaki E, Frilling A, Gerken G, Broelsch CE. CNI-sparing regimens within the liver transplant setting: experiences of a single center. Clin Transpl 2004;215-20.
Beckebaum S (b), Cicinnati VR, Broelsch CE. Future directions in immunosuppression. Transplant Proc 2004;36:574S-6S.
Beckebaum S, Cicinnati V, Gerken G. Current concepts for prophylaxis and treatment of hepatitis B reinfection after liver transplantation. Med Klin (Munich) 2008;103:190-7.
Beckebaum S, Sotiropoulos G, Gerken G, Cicinnati V. Hepatitis B and liver transplantation: 2008 update. Rev Med Virol 2009;19:7-29.
Beckebaum S, Iacob S, Klein CG, et al. Assessment of allograft fibrosis by transient elastography and noninvasive biomarker scoring systems in liver transplant patients. Transplantation 2010;89:983-93.
Beckebaum S, Cicinnati VR. Conversion to combined mycophenolate mofetil and low-dose calcineurin inhibitor therapy for renal dysfunction in liver transplant patients: never too late? Dig Dis Sci 2011;56:4-6.
Beckebaum S (a), Kabar I, Cicinnati VR. Hepatitis B and C in liver transplantation: new strategies to combat the enemies. Rev Med Virol 2013;23(3):172-93.
Beckebaum S (b), Cicinnati VR, Radtke A, Kabar I. Calcineurin inhibitors in liver transplantation – still champions or threatened by serious competitors? Liver Int 2013;33(5):656-65.
Beckebaum S (c), Iacob S, Radtke A, Kabar I, Cicinnati VR. Body weight may not be sufficient for reliable estimation of subcutaneous hepatitis B immunoglobulin dose requirement. American Journal of Transplantation 2013;13:1615-16.
Beckebaum S, Herzer K, Bauhofer A, et al. Recurrence of hepatitis B infection in liver transplant patients receiving long-term hepatitis B immunoglobulin prophylaxis. Ann Transplant 2018;23:789-801.
Becker T, Foltys D, Bilbao I, et al. Patient outcomes in two steroid-free regimens using tacrolimus monotherapy after daclizumab induction and tacrolimus with mycophenolate mofetil in liver transplantation. Transplantation 2008;86:1689-94.
Benlloch S, Berenguer M, Prieto M, et al. De novo internal neoplasms after liver transplantation: increased risk and aggressive behavior in recent years? Am J Transplant 2004;4:596-604.
Bergquist A, Weismüller TJ, Levy C, Rupp C et al; International PSC Study Group. Impact on follow-up strategies in patients with primary sclerosing cholangitis. Liver Int. 2023 Jan;43(1):127-138.
Bethea E, Arvind A, Gustafson J et al. Immediate administration of antiviral therapy after transplantation of hepatitis C-infected livers into uninfected recipients: Implications for therapeutic planning. Am J Transplant 2019. doi: 10.1111/ajt.15768. [Epub ahead of print].
Bhartia B, Ward J, Guthrie JA, Robinson PJ. Hepatocellular carcinoma in cirrhotic livers: double-contrast thin-section MR imaging with pathologic correlation of explanted tissue. AJR Am J Roentgenol 2003;180:577-84.
Bhat M, Mara K, Dierkhising R, Watt KD. Gender, race and disease aetiology predict de novo malignancy risk following liver transplantation: Insights for future individualised cancer screening guidance. Transplantation 2018. doi: 10.1097/TP.0000000000002113. [Epub ahead of print].
Bittermann T, Hoteit MA, Abt PL, Forde KA, Goldberg D. Waiting time and explant pathology in transplant recipients with hepatocellular carcinoma: a novel study using national data. Am J Transplant 2014;14:1657-63.
Borg BB, Feng Z, Earl TM, Anderson CD. Hepatitis E in post-liver transplantation: is it time to routinely consider it? Clin Transplant 2016;30(9):975-9.
Brandhagen DJ. Liver transplantation for hereditary haemochromatosis. Liver Transpl 2001;7:663-7.
Broelsch CE, Whitington PF, Emond JC, et al. Liver transplantation in children from living related donors. Surgical techniques and results. Ann Surg 1991;214:428-37.
Brüggenwirth IMA, Mueller M, Lantinga VA, et al. Prolonged preservation by hypothermic machine perfusion facilitates logistics in liver transplantation: A European observational cohort study. Am J Transplant. 2022 Jul;22(7):1842-1851.
Buchholz BM, Ferguson JW, Schnitzbauer AA, et al. Liver and kidney recipient selection of hepatitis C virus viremic donors: Meeting consensus report from the 2019 controversies in transplantation. Transplantation 2020; 104(3):478-81.
Buchholz BM, Ferguson JW, Schnitzbauer AA et al.; International SiLVER study group. Randomised Sirolimus-based Early Calcineurin Inhibitor Reduction in Liver Transplantation: Impact on Renal Function. Transplantation. 2020 May;104(5):1003-1018.
Buell JF, Gross TG, Woodle ES. Malignancy after transplantation.Transplantation 2005;15:S254-64.
Buis CI, Geuken E, Visser DS, Kuipers F, Haagsma EB, Verkade HJ, et al. Altered bile composition after liver transplantation is associated with the development of nonanastomotic biliary strictures. J Hepatol 2009;50:69-79.
Burra P, Senzolo M, Adam R, et al. Liver transplantation for alcoholic liver disease in Europe: a study from the ELTR (European Liver Transplant Registry). Am J Transplant 2010;10:138-48.
Burra P, Germani G. Orthotopic liver transplantation In non-alcoholic fatty liver disease patients. Rev Recent Clin Trials. 2014;9(3):210-6.
Burton JR Jr, Terrault NA, Goldberg DS, et al. Liver and kidney recipient selection of HCV-viremic donors - Meeting Consensus Report from the 2019 Controversies in Transplantation. Transplantation 2019. doi: 10.1097/TP.0000000000003014. [Epub ahead of print].
Buti M, Mas A, Prieto M, et al. Adherence to lamivudine after an early withdrawal of hepatitis B immune globulin plays an important role in the long-term prevention of hepatitis B virus recurrence. Transplantation 2007;84:650-4.
Bzeizi KI, Smith R, Albenmousa A, et al. Long-Term Outcomes of Everolimus Therapy in De Novo Liver Transplantation: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Transplant Proc. 2021 Jan-Feb;53(1):148-158.
Cai CJ, Lu MQ, Chen YH, Zhao H, Li MR, Chen GH. Clinical study on prevention of HBV re-infection by entecavir after liver transplantation. Clin Transplant. Clin Transplant 2012;26(2):208-15.
Cantarovich M, Tzimas GN, Barkun J, Deschênes M, Alpert E, Tchervenkov J. Efficacy of mycophenolate mofetil combined with very low-dose cyclosporine microemulsion in long-term liver-transplant patients with renal dysfunction. Transplantation 2003;76:98-102.
Carbone M, Neuberger J. Liver transplantation in PBC and PSC: indications and disease recurrence. Clin Res Hepatol Gastroenterol 2011;35:446-54.
Carbone M, Neuberger JM. Autoimmune liver disease, autoimmunity and liver transplantation. J Hepatol 2014;60(1):210-23.
Carenco C, Assenat E, Faure S, Duny Y, Danan G, Bismuth M, Herrero A, Jung B, Ursic-Bedoya J, Jaber S, Larrey D, Navarro F, Pageaux GP. Tacrolimus and the risk of solid cancers after liver transplant: a dose effect relationship. Am J Transplant 2015;15(3):678-86.
Carlisle EM, Testa G. Adult to adult living related liver transplantation: Where do we currently stand? World J Gastroenterol 2012 Dec 14;18(46):6729-36.
Carrique L, Quance J, Tan A, et al. Results of Early Transplantation for Alcohol-Related Cirrhosis: Integrated Addiction Treatment With Low Rate of Relapse. Gastroenterology. 2021 Dec;161(6):1896-1906.e2.
Corpechot C, Chazouillères O, Belnou P, et al; Global PBC Study Group. Long-term impact of preventive UDCA therapy after transplantation for primary biliary cholangitis. J Hepatol. 2020 Sep;73(3):559-565.
Caroti L, Zanazzi M, Paudice N, et al. Conversion from calcineurin inhibitors to everolimus with low-dose cyclosporine in renal transplant recipients with squamous cell carcinoma of the skin. Transplant Proc 2012;44(7):1926-27.
Chan G, Taqi A, Marotta P, et al. Long-term outcomes of emergency liver transplantation for acute liver failure. Liver Transpl 2009;15:1696-702.
Charatcharoenwitthaya P, Pimentel S, Talwalkar, et al. Long-term survival and impact of ursodeoxycholic acid treatment for recurrent primary biliary cirrhosis after liver transplantation. Liver Transpl 2007;13:1236-45.
Charlton M (a), Everson GT, Flamm SL, et al. Ledipasvir and sofosbuvir plus ribavirin for treatment of HCV infection in patients with advanced liver disease. Gastroenterology 2015; 149: 649–59.
Charlton M (b), Gane E, Manns MP et al. Sofosbuvir and ribavirin for treatment of compensated recurrent hepatitis C virus infection after liver transplantation. Gastroenterology 2015;148(1):108-17.
Charlton M (c), Levitsky J, Aqel B et al. International Liver Transplantation Society Consensus Statement on Immunosuppression in Liver Transplant Recipients. Transplantation 2019. doi: 10.1097/TP.0000000000002980.
Choi PC, Kim HJ, Choi WH, et al. Model for end-stage liver disease, model for end-stage liver disease-sodium and Child-Turcotte-Pugh scores over time for the prediction of complications of liver cirrhosis. Liver Int 2009;29:221-6.
Cholongitas E, Shusang V, Papatheodoridis GV, et al. Risk factors for recurrence of primary sclerosing cholangitis after liver transplantation. Liver Transpl 2008;14:138-43.
Cholongitas E, Goulis I, Antoniadis N, et al. New nucleos(t)ide analogue monoprophylaxis after cessation of hepatitis B immunoglobulin is effective against hepatitis B recurrence. Transpl Int 2014;27(10):1022-8.
Choudhary NS, Saigal S, Saraf N, Soin AS. Extrahepatic Malignancies and Liver Transplantation: Current Status. J Clin Exp Hepatol. 2021 Jul-Aug;11(4):494-500.
Cicinnati VR (a), Yu Z, Klein CG, et al. Clinical trial: switch to combined mycophenolate mofetil and minimal dose calcineurin inhibitor in stable liver transplant patients-assessment of renal and allograft function, cardiovascular risk factors and immune monitoring. Aliment Pharmacol Ther 2007;26:1195-208.
Copaci I, Lupescu I, Caceaune E, Chiriac G, Ismail G. Noninvasive Markers of improvement of liver steatosis achieved by weight reduction in patients with nonalcoholic fatty liver disease. Rom J Intern Med 2015;53(1):54-62.
Cornberg M, Protzer U, Petersen J, et al. Prophylaxis, diagnosis and therapy of hepatitis B virus infection – the German guideline. Z Gastroenterol 2011;49:871-930.
Cornberg M, Sandmann L, Protzer U et al.; Collaborators:. S3-Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) zur Prophylaxe, Diagnostik und Therapie der Hepatitis-B-Virusinfektion – (AWMF-Register-Nr. 021-11). Z Gastroenterol. 2021 Jul;59(7):691-776.
Cotter TG, Paul S, Sandıkçı B et al. Improved graft survival after liver transplantation for recipients with hepatitis C virus in the direct-acting antiviral era. Liver Transpl 2019;25(4):598-609. doi: 10.1002/lt.25424.
Cotter TG, Charlton M. Nonalcoholic steatohepatitis after liver transplantation. Liver Transpl 2020;26(1):141-59
Craig DG, Ford AC, Hayes PC, Simpson KJ. Systematic review: prognostic tests of paracetamol-induced acute liver failure. Aliment Pharmacol Ther 2010;31:1064-76.
Créput C, Blandin F, Deroure B, et al. Long-term effects of calcineurin inhibitor conversion to mycophenolate mofetil on renal function after liver transplantation. Liver Transpl 2007;13:1004-10.
Crismale JF, Khalid M, Bhansali A, et al. Liver, simultaneous liver-kidney, and kidney transplantation from hepatitis C positive donors in hepatitis C negative recipients: A single-center study. Clin Transplant 2019:e13761.
Cross T, Jothimani D, Heneghan MA, Harrison PM. Non-invasive assessment of fibrosis in liver grafts due to hepatitis C virus recurrence. Clin Transplant 2011;25:345-51.
Czigany Z, Pratschke J, Froněk J, et al. Hypothermic Oxygenated Machine Perfusion Reduces Early Allograft Injury and Improves Post-transplant Outcomes in Extended Criteria Donation Liver Transplantation From Donation After Brain Death: Results From a Multicenter Randomised Controlled Trial (HOPE ECD-DBD). Ann Surg. 2021 Nov 1;274(5):705-712.
Dar FS, Faraj W, Zaman MB, Bartlett A, et al. Outcome of liver transplantation in hereditary haemochromatosis. Transpl Int 2009;22:717-24.
Darwish Murad S, Metselaar HJ. The invasion of fatty liver disease in liver transplantation. Transpl Int 2015; 29(4):416-7.
Daswani R, Kumar A, Sharma P, Singla V, Bansal N, Arora A. Role of liver transplantation in severe alcoholic hepatitis. Clin Mol Hepatol 2018;24(1):43-50.
Demetris AJ, Murase N, Lee RG, et al. Chronic rejection. A general overview of histopathology and pathophysiology with emphasis on liver, heart and intestinal allografts. Ann Transplant 1997;2:27-44.
De Martin E, Coilly A, Chazouillères O, et al.; FILFOIE consortium – France. Early liver transplantation for corticosteroid non-responders with acute severe autoimmune hepatitis: The SURFASA score. J Hepatol. 2021 Jun;74(6):1325-1334.
Demetris AJ, Bellamy C, Hubscher SG, et al. 2016 Comprehensive update of the Banff Working Group on liver allograft pathology: introduction of antibody-mediated rejection. Am J Transplant 2016;16:2816-35.
De Simone P (a), Fagiuoli S, Cescon M, De Carlis L, Tisone G, Volpes R, Cillo U; Consensus Panel. Use of everolimus in liver transplantation: Recommendations from a Working Group. Transplantation 2016;101(2):239-251.
De Simone P (b), Romagnoli R, Tandoi F, et l. Early introduction of subcutaneous Hepatitis B Immunoglobulin following liver transplantation for Hepatitis B virus infection: A prospective, multicenter study. Transplantation 2016;100(7):1507-12.
Dew MA, DiMartini AF, Steel J, et al. Meta-analysis of risk for relapse to substance use after transplantation of the liver or other solid organs. Liver Transpl 2008;14:159-72.
Di Benedetto F, Tarantino G, Montalti R, et al. Sorafenib before liver transplantation for hepatocellular carcinoma: risk or give up. Transpl Int 2011;24(11):e97.
Dobrindt EM, Keshi E, Salim Y, et al. Hepatitis B Immunoglobulin discontinuation in long-term liver transplant patients. Transpl Infect Dis. 2020 Aug;22(4):e13303.
Duffy JP, Vardanian A, Benjamin E, et al. Liver transplantation criteria for hepatocellular carcinoma should be expanded: a 22-year experience with 467 patients at UCLA. Ann Surg 2007;246:502-9.
Dutkowski P, Oberkofler CE, Béchir M, et al. The model for end-stage liver disease allocation system for liver transplantation saves lives, but increases morbidity and cost: a prospective outcome analysis. Liver Transpl 2011;17:674-84.
Duvoux C, Roudot-Thoraval F, Decaens T, et al; Liver Transplantation French Study Group. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143(4):986-94.
EASL Clinical Practice Guidelines: Liver transplantation. J Hepatol 2016;64:433-85.https://dx.doi.org/10.1016/j.jhep.2015.10.006.
EASL recommendations on treatment of hepatitis C: Final update of the series. J Hepatol. 2020 Nov;73(5):1170-1218.
EASL Clinical Practise Guideline: Management of alcohol-related liver disease. J Hepatol 2018;69:154-81. https://www.easl.eu/medias/cpg/ Alcoholic-LiverDisease/2018/English-report.pdf.
EASL Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J Hepatol 2017;67:370-98. https://www.easl.eu/medias/ cpg/management-of-hepatitis-B-virus-infection/English-report.pdf.
EASL Clinical Practical Guidelines on the Management of Acute (Fulminant) Liver Failure. J Hepatol 2017;66:1047-81. https://www.easl.eu/ medias/cpg/ALF/English-report.pdf
EASL Clinical Practice Guidelines on hepatitis E virus infection. J Hepatol 2018;68:1256-1271. https://www.journal-of-hepatology.eu/article/ S0168-8278(18)30155-7/pdf.
Ecker BL, Hoteit MA, Forde KA, et al. Patterns of discordance between pretransplant imaging stage of hepatocellular carcinoma and posttransplant pathologic stage: A contemporary appraisal of the milan criteria. Transplantation 2018;102(4):648-55.
Egawa H, Sakisaka S, Teramukai S, Sakabayashi S, Yamamoto M, Umeshita K, Uemoto S. Long-term outcomes of living-donor liver transplantation for primary biliary cirrhosis: A Japanese multicenter Study. Am J Transplant 2016;16(4):1248-57.
Eid AJ, Razonable RR. New developments in the management of cytomegalovirus infection after solid organ transplantation. Drugs 2010;70:965-81.
Euvrard S, Morelon E, Rostaing L, et al. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 2012;367(4):329-9.
Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore SJ. Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients. Cochrane Database Syst Rev 2018;9:4:CD007606. doi: 10.1002/14651858. CD007606.pub4. [Epub ahead of print].
Faisal N, Renner EL. Recurrence of autoimmune liver diseases after liver transplantation. World J Hepatol 2015; 7(29): 2896–2905.
Fairfield C, Penninga L, Powell J, Harrison EM, Wigmore SJ. Glucocorticosteroid-free versus glucocorticosteroid-containing immunosuppression for liver transplanted patients. Cochrane Database Syst Rev 2018;4:CD007606.
Feng S, Goodrich NP, Bragg-Gresham JL, et al. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant 2006;6:783-90.
Ferrarese A, Morelli MC, Carrai P, et al. Outcomes of Liver Transplant for Adults With Wilson's Disease. Liver Transpl. 2020 Apr;26(4):507-516.
Fevery J, Henckaerts L, Van Oirbeek R, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int 2012; 32:214-22.
Filipponi F, Callea F, Salizzoni M, et al. Double-blind comparison of hepatitis C histological recurrence rate in HCV+ liver transplant recipients given basiliximab + steroids or basiliximab + placebo, in addition to cyclosporine and azathioprine. Transplantation 2004;78:1488-95.
Finkenstedt A, Graziadei IW, Oberaigner W, et al. Extensive surveillance promotes early diagnosis and improved survival of de novo malignancies in liver transplant recipients. Am J Transplant 2009; 9:2355-61.
Fischer L, Klempnauer J, Beckebaum S, et al. A randomised, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation-PROTECT. Am J Transplant 2012;12:1855-65.
Fontana RJ, Ellerbe C, Durkalski VE, et al. Two-year outcomes in initial survivors with acute liver failure: results from a prospective, multicenter study. Liver Int 2015;35(2):370-80. doi: 10.1111/liv.12632. Epub 2014 Jul 28.
Frilling A, Malago M, Weber F, et al. Liver transplantation for patients with metastatic endocrine tumours. Liver Transpl 2006;12:1089-96.
Fung JJ, Jain A, Kwak EJ, Kusne S, Dvorchik I, Eghtesad B. De novo malignancies after liver transplantation: a major cause of late death. Liver Transpl 2001;7:S109-18.
Fung J, Cheung C, Chan SC, Yuen MF. Entecavir monotherapy is effective in suppressing hepatitis B virus after liver transplantation. Gastroenterology 2011;141:1212-9.
Fung JY. Liver transplantation for severe alcoholic hepatitis-The CON view. Liver Int 2017;37(3):340--2. doi: 10.1111/liv.13286.
Fung J, Mak LY, Chan AC, et al. Model for end-stage liver disease with additional criteria to predict short-term mortality in severe flares of chronic hepatitis B. Hepatology 2019. doi: 10.1002/hep.31086. [Epub ahead of print].
Gane E, Strasser SI, Patterson S, et al. A prospective study on the safety and efficacy of lamivudine and adefovir dipivoxil prophylaxis in HBsAg positive liver transplantation candidates. Hepatology 2007;46:479A.
Gane EJ, Patterson S, Strasser SI, McCaughan GW, Angus PW. Combination of lamivudine and adefovir without hepatitis B immune globulin is safe and effective prophylaxis against hepatitis B virus recurrence in hepatitis B surface antigen positive liver transplant candidates. Liver Transpl 2013;19(3):268-74.
Gane EJ, Robson RA, Bonacini M et al. Safety, anti-viral efficacy and pharmacokinetics (PK) of sofosbuvir (SOF) in patients with severe renal impairment. Hepatology 2014;60:667a-667.
Garcia CE, Ribeiro HB, Garcia RL, et al. Mycophenolate mofetil in stable liver transplant patients with calcineurin inhibitor-induced renal impairment: single-center experience. Transplant Proc 2003;35:1131-2.
Gedaly R, Daily MF, Davenport D, et al. Liver transplantation for the treatment of liver metastases from neuroendocrine tumours: an analysis of the UNOS database. Arch Surg 2011;146:953-8.
Geissler EK, Schnitzbauer AA, Zülke C, et al. Sirolimus use in liver transplant recipients with hepatocellular carcinoma: A randomised, multicenter, open-label phase 3 trial. Transplantation 2016;100(1):116-25.
Giard JM, Dodge JL, Terrault N. Superior wait-list outcomes in patients with alcohol-associated liver disease compared to other indications for liver transplantation. Liver Transpl 2019. doi: 10.1002/lt.25485. [Epub ahead of print].
Gonwa TA, Mai ML, Melton LB, Hays SR, et al. End-stage renal disease (ESRD) after orthotopic liver transplantation (OLTX) using calcineurin-based immunotherapy: risk of development and treatment. Transplantation 2001;72:1934-9.
Gonzalez-Koch A, Czaja AJ, Carpenter HA, et al. Recurrent autoimmune hepatitis after orthotopic liver transplantation. Liver Transpl 2001;7:302-10.
Goss JA, Shackleton CR, Farmer DG, et al. Orthotopic liver transplantation for primary sclerosing cholangitis. A 12-year single center experience. Ann Surg 1997;225:472-81.
Graziadei IW, Wiesner RH, Marotta PJ, et al. Long-term results of patients undergoing liver transplantation for primary sclerosing cholangitis. Hepatology 1999;30:1121-7.
Graziadei IW, Schwaighofer H, Koch R, et al. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl 2006;12:718-25.
Graziadei IW, Zoller H, Fickert P, et al. Indications for liver transplantation in adults: Recommendations of the Austrian Society for Gastroenterology and Hepatology (ÖGGH) in cooperation with the Austrian Society for Transplantation, Transfusion and Genetics (ATX). Wien Klin Wochenschr 2016;128(19-20):679-90.
Gross GE, Eisert L, Doerr HW, et al. S2k-Leitlinie zur Diagnostik und Therapie des Zoster und der Postzosterneuralgie. J Dtsch Dermatol Ges. 2020 Jan;18(1):55-79.
Gu YH, Du JX, Ma ML. Sirolimus and non-melanoma skin cancer prevention after kidney transplantation: A meta-analysis. Asian Pac J Cancer Prev 2012;13(9):4335-9.
Guba M, von Breitenbuch P, Steinbauer M, et al. Rapamycin inhibits primary and metastatic tumour growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med 2002;8:128-35.
Guichelaar MM, Kendall R, Malinchoc M, Hay JE. Bone mineral density before and after OLT: long-term follow-up and predictive factors. Liver Transpl 2006;12(9):1390-402.
Guichelaar MM, Schmoll J, Malinchoc M, Hay JE. Fractures and avascular necrosis before and after orthotopic liver transplantation: longterm follow-up and predictive factors. Hepatology 2007;46(4):1198-207.
Guillaud O, Jacquemin E, Couchonnal E, et al. Long term results of liver transplantation for alpha-1 antitrypsin deficiency. Dig Liver Dis. 2021 May;53(5):606-611.
Haga H, Miyagawa-Hayashino A, Taira K, Morioka D, Egawa H, Takada Y, Manabe T, Uemoto S. Histological recurrence of autoimmune liver diseases after living-donor liver transplantation. Hepatol Res 2007;37 Suppl 3:S463–S69.
Hanouneh IA, Macaron C, Lopez R. Risk of colonic neoplasia after liver transplantation for primary sclerosing cholangitis. Inflamm Bowel Dis 2012;18:269-74.
Harms MH, van Buuren HR, Corpechot C, et al.
Ursodeoxycholic acid therapy and liver transplant-free survival in patients with primary biliary cholangitis. J Hepatol 2019;71(2):357-65.
Harper SJ, Gelson W, Harper IG, Alexander GJ, Gibbs P. Switching to sirolimus-based immune suppression after liver transplantation is safe and effective: a single-center experience. Transplantation 2011;91:128-32.
Hashimoto E, Shimada M, Noguchi S, et al. Disease recurrence after living liver transplantation for primary biliary cirrhosis: a clinical and histological follow-up study. Liver Transpl 2001;7:588-95.
Heimbach JK. Successful liver transplantation for hilar cholangiocarcinoma. Curr Opin Gastroenterol. 2008;24:384-8.
Hernandez Mdel P, Martin P, Simkins J. Infectious complications after liver transplantation. Gastroenterol Hepatol 2015;11(11):741-53.
Hiatt JR, Carmody IC, Busuttil RW. Should we expand the criteria for hepatocellular carcinoma with living-donor liver transplantation?-no, never. J Hepatol 2005;43:573-7.
Hinz M, Wree A, Jochum C et al High age and low sodium urine concentration are associated with poor survival in patients with hepatorenal syndrome. Ann Hepatol 2013;12(1):92-9.
Ho GT, Seddon AJ, Therapondos G, Satsangi J, Hayes PC. The clinical course of ulcerative colitis after orthotopic liver transplantation for primary sclerosing cholangitis: further appraisal of immunosuppression post transplantation. Eur J Gastroenterol Hepatol 2005;17:1379-85.
Höfer A, Jonigk D, Hartleben B, et al. DSA Are Associated With More Graft Injury, More Fibrosis, and Upregulation of Rejection-associated Transcripts in Subclinical Rejection. Transplantation. 2020 Mar;104(3):551-561.
Hong JC, Jones CM, Duffy JP, et al. Comparative analysis of resection and liver transplantation for intrahepatic and hilar cholangiocarcinoma: a 24-year experience in a single center. Arch Surg 2011;146:683-9.
Hoyos S, Escobar J, Cardona D, et al. Factors associated with recurrence and survival in liver transplant patients with HCC – a single center retrospective study. Ann Hepatol 2015;14(1):58-63.
Hüsing A, Cicinnati VR, Beckebaum S et al. Endoscopic ultrasound: valuable tool for diagnosis of biliary complications in liver transplant recipients? Surg Endosc 2015;29(6):1433-8.
Hüsing A (a), Schmidt M, Beckebaum S, Cicinnati VR, Koch R, Thölking G, Stella J, Heinzow H, Schmidt HH, Kabar I. Long-term renal function in liver transplant recipients after conversion from calcineurin inhibitors to mTOR Inhibitors. Ann Transplant 2015 26;20:707-13.
Hüsing A (b), Cicinnati VR, Maschmeier M, Schmidt HH, Wolters HH, Beckebaum S, Kabar I. Complications after endoscopic sphincterotomy in liver transplant recipients: A retrospective single-centre study. Arab J Gastroenterol 2015;16(2):46-9.
Hytiroglou P, Gutierrez JA, Freni M, et al. Recurrence of primary biliary cirrhosis and development of autoimmune hepatitis after liver transplant: A blind histologic study. Hepatol Res 2009;39:577-84.
Iacob S, Cicinnati VR, Dechêne A, et al. Genetic, immunological and clinical risk factors for biliary strictures following liver transplantation. Liver Int 2012;32(8):1253-61.
Iida T, Ogura Y, Oike F, Hatano E, Kaido T, Egawa H, Takada Y, Uemoto S. Surgery-related morbidity in living donors for liver transplantation. Transplantation 2010;89:1276–82.
Im GY, Kim-Schluger L, Shenoy A, et al. Early liver transplantation for severe alcoholic hepatitis in the United States-A single-center experience. Am J Transplant 2016;16(3):841-9. doi: 10.1111/ajt.13586.
Im GY, Cameron AM, Lucey MR. Liver transplantation for alcoholic hepatitis. J Hepatol 2019;70(2):328-34.
Invernizzi F, Iavarone M, Zavaglia C, et al.
Experience with early sorafenib treatment with mTOR inhibitors in hepatocellular carcinoma recurring after liver transplantation. Transplantation 2019 doi: 10.1097/TP.0000000000002955. [Epub ahead of print].
Jadlowiec CC, Morgan PE, Nehra AK, et al. Not All Cellular Rejections Are the Same: Differences in Early and Late Hepatic Allograft Rejection. Liver Transpl. 2019 Mar;25(3):425-435.
Jelic S, Sotiropoulos GC; ESMO Guidelines Working Group. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:59-64.
Jimenez C, Marques E, Manrique A, al. Incidence and risk factors of development of lung tumours after liver transplantation. Transplant Proc 2005;37:3970-2.
Jørgensen KK, Lindström L, Cvancarova M, et al. Colorectal neoplasia in patients with primary sclerosing cholangitis undergoing liver transplantation: a Nordic multicenter study. Scand J Gastroenterol 2012;47(8-9):1021-9.
Jørgensen KK, Lindström L, Cvancarova M et al. Immunosuppression after liver transplantation for primary sclerosing cholangitis influences activity of inflammatory bowel disease. Clin Gastroenterol Hepatol 2013;11(5):517-23.
John PR, Thuluvath PJ. Outcome of patients with new-onset diabetes mellitus after liver transplantation compared with those without diabetes mellitus. Liver Transpl 2002;8:708-13.
Kahn J, Wagner D, Homfeld N, Müller H, Kniepeiss D, Schemmer P. Both sarcopenia and frailty determine suitability of patients for liver transplantation-A systematic review and meta-analysis of the literature. Clin Transplant 2018;32(4):e13226. doi: 10.1111/ctr.13226. [Epub ahead of print].
Kaltenborn A, Salinas R, Jäger MD, Lehner F, Sakirow L, Klempnauer J, Schrem H. Model of end-stage liver disease score and derived variants lack prognostic ability after liver transplantation. Ann Transplant 2015;20:441-8.
Kamar N (a), Abravanel F, Selves J, et al. Influence of immunosuppressive therapy on the natural history of genotype 3 hepatitis-E virus infection after organ transplantation. Transplantation 2010;89(3):353-60.
Kamar N (b), Rostaing L, Abravanel F, et al. Ribavirin therapy inhibits viral replication on patients with chronic hepatitis e virus infection. Gastroenterology 2010.;139(5):1612-8.
Kamar N (c) Rostaing L, Abravanel F, et al. Pegylated interferon-alpha for treating chronic hepatitis E virus infection after liver transplantation. Clin Infect Dis 2010;50(5):e30-e3.
Kamar N, Garrouste C, Haagsma EB, et al. Factors associated with chronic hepatitis in patients with hepatitis E virus infection who have received solid organ transplants. Gastroenterology 2011;140(5):1481-9.
Kamar N, Izopet J, Tripon S, Bismuth M, Hillaire S, Dumortier J, Radenne S, Coilly A, Garrigue V, D’Alteroche L, Buchler M, Couzi L, Lebray P, Dharancy S, Minello A, Hourmant M, Roque-Afonso AM, Abravanel F, Pol S, Rostaing L, Mallet V. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N Engl J Med 2014;370(12):1111-20.
Kamdar KY, Rooney CM, Heslop HE. Posttransplant lymphoproliferative disease following liver transplantation. Curr Opin Organ Transplant 2011;16:274-80.
Kang I, Lee JG, Choi SH, et al. Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin Mol Hepatol. 2021 Oct;27(4):589-602.
Kapoor D, Guptan RC, Wakil SM, et al. Beneficial effects of lamivudine in hepatitis B virus-related decompensated cirrhosis. J Hepatol 2000;33:308-12.
Kapoor VK. Bile duct injury repair – earlier is not better. Front Med 2015:9(4):508-11.
Kasturi KS, Chennareddygari S, Mummadi RR. Effect of bisphosphonates on bone mineral density in liver transplant patients: a metaanalysis and systematic review of randomised controlled trials. Transpl Int 2010;23(2):200-7.
Kawahara T, Asthana S, Kneteman NM. m-TOR inhibitors: What role in liver transplantation? J Hepatol 2011;55(6):1441-51.
Kawasaki S, Makuuchi M, Matsunami H, et al. Living related liver transplantation in adults. Ann Surg 1998;227:269-74.
Kemmer N, Kaiser T, Zacharias V, Neff GW. Alpha-1-antitrypsin deficiency: outcomes after liver transplantation. Transplant Proc 2008;40:1492-4.
Kim JM, Kwon CH, Joh JW, Ha YE, Sinn DH, Choi GS, Peck KR, Lee SK. Oral valganciclovir as a preemptive treatment for cytomegalovirus (CMV) Infection in CMV-seropositive liver transplant recipients. PLoS One 2015; 10(5):e0123554.doi: 10.1371/journal.pone.0123554. eCollection 2015.
Kneteman NM, Oberholzer J, Al Saghier M, et al. Sirolimus-based immunosuppression for liver transplantation in the presence of extended criteria for hepatocellular carcinoma. Liver Transpl 2004;10:1301-11.
Knight SR, Friend PJ, Morris PJ. Role of transplantation in the management of hepatic malignancy. Br J Surg 2007;94:1319-30.
Knighton S, Agarwal K, Heneghan MA, et al. A pharmacist delivered stratified conversion protocol from hepatitis B immunoglobulin (HBIG) to tenofovir or entecavir is efficacious, safe and cost-effective for prevention of recurrence of hepatitis B virus (HBV) in liver transplant (LT) recipients. Hepatology 2012;54:619A.
Konrad T, Steinmüller T, Vicini P, et al. Regulation of glucose tolerance in patients after liver transplantation: impact of cyclosporin versus tacrolimus therapy. Transplantation 2000;69:2072-8.
Kotton CN, Kumar D, Caliendo AM, et al.; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the Management of Cytomegalovirus in Solid-organ Transplantation. Transplantation 2018;102(6):900-31. doi: 10.1097/TP.0000000000002191.
Krinsky GA, Lee VS, Theise ND, et al. Transplantation for hepatocellular carcinoma and cirrhosis: sensitivity of magnetic resonance imaging. Liver Transpl 2002;8:1156-64.
Kubiliun M, Patel SJ, Hur C, Dienstag JL, Luther J. Early liver transplantation for alcoholic hepatitis: Ready for primetime? J Hepatol 2018;68(3):380-2.
Kulik L, Heimbach JK, Zaiem F et al. Therapies for patients with hepatocellular carcinoma awaiting liver transplantation: A systematic review and meta-analysis. Hepatology 2018 ;67(1):381-400.
Kwong AJ, Lai JC, Dodge JL, Roberts JP. Outcomes for liver transplant candidates listed with low model for end-stage liver disease score. Liver Transpl 2015;21(11):1403-9.
Kwong A, Kim WR, Mannalithara A, Heo NY, Udompap P, Kim D. Decreasing mortality and disease severity in hepatitis C patients awaiting liver transplantation in the United States. Liver Transpl 2017. doi: 10.1002/lt.24973. [Epub ahead of print].
Lange CM, Bojunga J, Hofmann WP, et al. Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 2009; 50:2001-6.
Lee BP (a), Terrault NA. Early liver transplantation for severe alcoholic hepatitis: moving from controversy to consensus. Curr Opin Organ Transplant 2018;23(2):229-36.
Lee BP (b), Vittinghoff E, Hsu C, et al. Predicting low-risk for sustained alcohol use after early liver transplant for acute alcoholic hepatitis: The sustained alcohol use post-liver transplant score. Hepatology 2019;69(4):1477-87.
Lee BP (c), Mehta N, Platt L, et al. Outcomes of early liver transplantation for patients with severe alcoholic hepatitis. Gastroenterology 2018;155(2):422-30.e1.
Lee BP (d), Im GY, Rice JP, et al. Patterns of Alcohol Use After Early Liver Transplantation for Alcoholic Hepatitis. Clin Gastroenterol Hepatol. 2022 Feb;20(2):409-418.e5.
Lee BP (e), Roth N, Rao P, et al. Artificial intelligence to identify harmful alcohol use after early liver transplant for alcohol-associated hepatitis. Am J Transplant. 2022 Jul;22(7):1834-1841.
Legrand-Abravanel F, Kamar N, Sandres-Saune K, Garrouste C, Dubois M, Mansuy JM, Muscari F, Sallusto F, Rostaing L, Izopet J. Characteristics of autochthonous hepatitis E virus infection in solid-organ transplant recipients in France. J Infect Dis 2010;202(6):835-44.
Leithead JA, Ferguson JW, Bates CM, Davidson JS, Simpson KJ, Hayes PC. Chronic kidney disease after liver transplantation for acute liver failure is not associated with perioperative renal dysfunction. Am J Transplant 2011;11:1905-15.
Lerut J, Bonaccorsi-Riani E, Finet P, Gianello P. Minimisation of steroids in liver transplantation. Transpl Int 2009; 22:2-19.
Levitsky J, Thudi K, Ison MG, Wang E, Abecassis M. Alemtuzumab induction in non-hepatitis C positive liver transplant recipients. Liver Transpl 2011;17:32-7.
Levitsky (b) J, Formica RN, Bloom RD, et al. The American Society of Transplantation Consensus Conference on the Use of Hepatitis C Viremic Donors in Solid Organ Transplantation. Am J Transplant. 2017 Nov;17(11):2790-2802.
Liang W, Wang D, Ling X, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: A meta-analysis. Liver Transpl 2012;18(1):62-9.
Liang HR, Hsieh CE, Lin KH. Living donor liver transplantation for hepatocellular carcinoma beyond the Milan criteria: outcome of expanded criteria in tumour size. BMC Surg. 2021 Nov 19;21(1):401.
Liermann Garcia RF, Evangelista Garcia C, McMaster P, Neuberger J. Transplantation for primary biliary cirrhosis: retrospective analysis of 400 patients in a single center. Hepatology 2001;33:22-7.
Lim WH, Ng CH, Ow ZGW, et al. A systematic review and meta-analysis on the incidence of osteoporosis and fractures after liver transplant. Transpl Int. 2021 Jun;34(6):1032-1043.
Lin M, Mittal S, Sahebjam F, Rana A, Sood GK. Everolimus with early withdrawal or reduced-dose Calcineurin-Inhibitors improves renal function in Liver Transplant Recipients: A systematic review and meta-analysis. Clin Transplant 2016, doi: 10.1111/ctr.12872. [Epub ahead of print].
Lin S, Hall A, Kumar R, Quaglia A, Jalan R. Validation of the SURFASA score to define steroid responsiveness in patients with acute autoimmune hepatitis. J Hepatol. 2022 Feb;76(2):485-487.
Lindström L, Jørgensen KK, Boberg KM et al. Risk factors and prognosis for recurrent primary sclerosing cholangitis after liver transplantation: a Nordic Multicentre Study. Scand J Gastroenterol. 2018 Mar;53(3):297-304.
Lisotti A, Fusaroli P, Caletti G. Role of endoscopy in the conservative management of biliary complications after deceased donor liver transplantation. World J Hepatol 2015;7(30):2927-32.
Liu X, Ling Z, Li L, Ruan B. Invasive fungal infections in liver transplantation. Int J Infect Dis 2011;15:e298-304.
Llado L, Fabregat J, Castellote J, et al. Impact of immunosuppression without steroids on rejection and hepatitis C virus evolution after liver transplantation: results of a prospective randomised study. Liver Transpl 2008;14:1752-60.
Lo CM, Fan ST, Liu CL, et al. Lessons learned from one hundred right lobe living donor liver transplants. Ann Surg 2004;240:151-8.
Lo CM, Liu CL, Lau GK, Chan SC, Ng IO, Fan ST. Liver transplantation for chronic hepatitis B with lamivudine-resistant YMDD mutant using add-on adefovir dipivoxil plus lamivudine. Liver Transpl 2005;11:807-13.
Lo CM. Liver transplantation for acute liver failure: not too early but never too late. Liver Transpl 2008;14:1243-4.
Lucey MR. Liver transplantation for severe alcoholic hepatitis - The PRO view. Liver Int 2017;37(3):343-344.
Machairas N, Kostakis ID, Tsilimigras DI, Prodromidou A, Moris D.
Liver transplantation for hilar cholangiocarcinoma: A systematic review. Transplant Rev (Orlando) 2020;34(1):100516.
Malhotra N, Beaton MD. Management of non-alcoholic fatty liver disease in 2015. World J Hepatol 2015;7(30):2962-7.
Manns M, Samuel D, Gane EJ et al. Ledipasvir and sofosbuvir plus ribavirin in patients with genotype 1 or 4 hepatitis C virus infection and advanced liver disease: a multicenter, open-label, randomised, phase 2 trial. Lancet Infect Dis 2016;16(6):685-97.
Markakis GE, Papatheodoridis GV, Cholongitas E. Epidemiology and treatment of hepatitis E in the liver transplantation setting: A literature review. J Viral Hepat. 2022 Sep;29(9):698-718.
Marroni CA. Management of alcohol recurrence before and after liver transplantation. Clin Res Hepatol Gastroenterol 2015 Sep;39 Suppl 1:S109-14.
Marroni CA, Fleck AM Jr, Fernandes SA, Galant LH, Mucenic M, de Mattos Meine MH, Mariante-Neto G, Brandão ABM. Liver transplantation and alcoholic liver disease: History, controversies, and considerations. World J Gastroenterol 2018;24(26):2785-805.
Marot A, Dubois M, Trépo E, Moreno C, Deltenre P. Liver transplantation for alcoholic hepatitis: A systematic review with meta-analysis. PLoS One 2018;13(1):e0190823. doi: 10.1371/journal.pone.0190823
Martin AP, Bartels M, Redlich J, Hauss J, Fangmann J. A single center experience with liver transplantation for Wilson’s disease. Clin Transplant 2008;22:216-21.
Martin P, DiMartini A, Feng S, Brown R, Jr, Fallon M. AASLD PRACTICE GUIDELINE. Evaluation for liver transplantation in adults: 2013 Practice Guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Hepatology 2014;59(3):1144-1165.
Massie AB, Caffo B, Gentry SE, Hall EC. MELD exceptions and rates of waiting list outcomes. Am J Transplant 2011;11:2362-71.
Masuoka HC, Rosen CB. Transplantation for cholangiocarcinoma. Clin Liver Dis 2011;15:699-715.
Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med 2011;365(19):1790-1800.
Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 model for analysis of competing risks of death after liver transplantation for hepatocellular carcinoma. Gastroenterology 2018;154(1):128-39.
McKenna M, Epperla N, Ghobadi A, et al. Real-world evidence of the safety and survival with CD19 CAR-T cell therapy for relapsed/refractory solid organ transplant-related PTLD. Br J Haematol. 2023 Jul;202(2):248-255
McPherson S, Elsharkawy AM, Ankcorn M, Ijaz S, Powell J, Rowe I, Tedder R, Andrews PA8. Summary of the British Transplantation Society UK Guidelines for Hepatitis E and Solid Organ Transplantation. Transplantation 2018; 102(1):15-20.
Meeks AC, Madill J. Sarcopenia in liver transplantation: A review. Clin Nutr ESPEN 2017;22:76-80.Memon K, Lewandowski RJ, Riaz A, Salem R. Yttrium 90 microspheres for the treatment of hepatocellular carcinoma. Recent Results Cancer Res 2013;190:207-24.
Merion RM, Schaubel DE, Dykstra DM, et al. The survival benefit of liver transplantation. Am J Transplant 2005;5:307-13.
Merli M, Giannelli V, Gentili F, et al. Ribavirin priming improves the virological response to antiviral treatment in transplanted patients with recurrent hepatitis C: a pilot study. Antivir Ther 2011;16:879-85.
Mitchell MC, Maddrey WC. Changing Times in Liver Transplantation for alcohol-associated liver disease. JAMA Intern Med 2019;179(3):348-350.
Moini M, Mistry P, Schilsky ML. Liver transplantation for inherited metabolic disorders of the liver. Curr Opin Organ Transplant 2010;15:269-76.
Molmenti EP, Netto GJ, Murray NG, et al. Incidence and recurrence of autoimmune/alloimmune hepatitis in liver transplant recipients. Liver Transpl 2002;8:519-26.
Montano-Loza AJ, Wasilenko S, Bintner J, Mason AL. Cyclosporine A protects against primary biliary cirrhosis recurrence after liver transplantation. Am J Transplant 2010;10:852-8.
Montano-Loza AJ, Bhanji RA, Wasilenko S, Mason AL. Systematic review: recurrent autoimmune liver diseases after liver transplantation. Aliment Pharmacol Ther 2016;45:4:485-500.
Montano-Loza AJ, Ronca V, Ebadi M, et al; International Autoimmune Hepatitis Group (IAIHG). Risk factors and outcomes associated with recurrent autoimmune hepatitis following liver transplantation. J Hepatol. 2022 Jul;77(1):84-97.
Factors associated with recurrence of primary biliary cholangitis after liver transplantation and effects on graft and patient survival. Gastroenterology 2019;156(1):96-107.e1.
Moreira Kulak CA, Cochenski B, Borba VZ, Kulak J, et al. Posttransplantation osteoporosis. Arq Bras Endocrinol Metab 2010;54:143-9.
Moreno Planas JM, Cuervas-Mons Martinez V, Rubio Gonzalez E, et al. Mycophenolate mofetil can be used as monotherapy late after liver transplantation. Am J Transplant 2004;4:1650-5.
Morioka D (a), Egawa H, Kasahara M, et al. Impact of human leukocyte antigen mismatching on outcomes of living donor liver transplantation for primary biliary cirrhosis. Liver Transpl 2007;13:80-90.
Morioka D (b), Egawa H, Kasahara M, et al. Outcomes of adult-to-adult living donor liver transplantation. A single institution`s experience with 335 consecutive cases. Ann Surg 2007;245:315-25.
Morris PD, Laurence JM, Yeo D, Crawford M, et al. Can response to locoregional therapy help predict long-term survival after liver transplantation for hepatocellular carcinoma? A systematic review. Liver Transpl. 2016 Dec 22. doi: 10.1002/lt.24689.
Mullen KD, Sanyal AJ, Bass NM, et al. Rifaximin is safe and well tolerated for long-term maintenance of remission from overt hepatic encephalopathy. Clin Gastroenterol Hepatol 2014;12(8):1390-7.
Mumtaz K, Faisal N, Husain S, et al. Universal prophylaxis or preemptive strategy for cytomegalovirus disease after liver transplantation: A systematic review and meta-analysis. Am J Transplant 2015;15(2):472-81.
Nadalin S, Capobianco I, Königsrainer I, Harder B, Königsrainer A. Living liver donor: indications and technical aspects. Chirurg. 2015;86(6):609-21.
Naoumov NV, Lopes AR, Burra P, et al. Randomised trial of lamivudine versus hepatitis B immunoglobulin for long-term prophylaxis of hepatitis B recurrence after liver transplantation. J Hepatol 2001;34:888-94.
Nangia G, Borges K, Reddy KR. Use of HCV-infected organs in solid organ transplantation: An ethical challenge but plausible option. J Viral Hepat 2019;26(12):1362-71.
Nel JD, Epstein S. Metabolic Bone Disease in the Posttransplant Population: Preventative and Therapeutic Measures. Med Clin North Am 2016;100(3):569-86.
Neuberger J. Incidence, timing, and risk factors for acute and chronic rejection. Liver Transpl Surg 1999;5:S30-6.
Notarpaolo A, Layese R, Magistri P, et al. Validation of the AFP model as a predictor of HCC recurrence in patients with viral hepatitisrelated cirrhosis who had received a liver transplant for HCC. J Hepatol 2017;66(3):552-559.
Okumura K, Yamashita T, Masuda T, et al. Long-term outcome of patients with hereditary transthyretin V30M amyloidosis with polyneuropathy after liver transplantation.Amyloid. 2016;23(1):39-45.
Orfanidou A, Papatheodoridis GV, Cholongitas E. Antiviral prophylaxis against hepatitis B recurrence after liver transplantation: Current concepts. Liver Int. 2021 Jul;41(7):1448-1461.
Pabón V, Dumortier J, Gincul R, et al. Long-term results of liver transplantation for Wilson’s disease. Gastroenterol Clin Biol 2008;32:378-81.
Parrish NF, Feurer ID, Matsuoak LK, Rega SA, Perri R, Alexopoulos SP. The changing face of liver transplantation in the United States: The effect of HCV antiviral eras on transplantation trends and and outcomes. Transplantation Direct 2019;5(3):pe427.
Perney P, Bismuth M, Sigaud H, et al. Are preoperative patterns of alcohol consumption predictive of relapse after liver transplantation for alcoholic liver disease? Transpl Int 2005;18(11):1292-129-7.
Pascher A, Sauer IM, Walter M, et al. Donor evaluation, donor risks, donor outcome, and donor quality of life in adult-to-adult living donor liver transplantation. Liver Transpl 2002;8:829-37.
Pascher A, Neuhaus P. Bile duct complications after liver transplantation. Transpl Int 2005;18:627-42.
Patel S, Orlaoff M, Tsoulfas G, et al. Living donor liver transplantation in the United States: identifying donors at risk for perioperative complications. Am J Transplant 2007;7:2344-9.
Paya C, Humar A, Dominguez E, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant 2004;4:611-20.
Perkins JD. Biliary tract complications: The most common postoperative complication in living liver donors. Liver Transpl 2008:14:1372-7.
Perney P, Bismuth M, Sigaud H, et al. Are preoperative patterns of alcohol consumption predictive of relapse after liver transplantation for alcoholic liver disease? Transpl Int 2005;18:1292-7.
Pereira P, Peixoto A. Biliary Complications - The “achilles heel” of orthotopic liver transplantation. J Gastroenterol 2018;25(1):1-3.
Pfitzmann R, Schwenzer J, Rayes N, Seehofer D, Neuhaus R, Nüssler NC. Long-term survival and predictors of relapse after orthotopic liver transplantation for alcoholic liver disease. Liver Transpl 2007;13:197-205.
Pischke S, Suneetha PV, Baechlein C, et al. Hepatitis E virus infection as a cause of graft hepatitis in liver transplant recipients. Liver Transpl 2010;16(1):74-82.
Poujois A, Sobesky R, Meissner WG, et al. Liver transplantation as a rescue therapy for severe neurologic forms of Wilson disease. Neurology. 2020 May 26;94(21):e2189-e2202.
Protzer U, Böhm F, Longerich T, et al. Molecular detection of hepatitis E virus (HEV) in liver biopsies after liver transplantation. Mod Pathol 2015;28(4):523-32.
Pulitano C, Ho P, Verran D, et al. Molecular profiling of post-reperfusion milieu determines acute kidney injury after liver transplantation: a prospective study. Liver Transpl 2018; 23. doi: 10.1002/lt.25178. [Epub ahead of print].
Qi HL, Zhuang BJ, Li CS, Liu QY. Peri-operative use of sorafenib in liver transplantation: a time-to-event meta-analysis. World J Gastroenterol 2015;21(5):1636-40.
Qin YS, Li ZS, Sun ZX, Wu RP, Wang N, Yao YZ. Endoscopic management of biliary complications after orthotopic liver transplantation. Hepatobiliary Pancreat Dis Int 2006;5:39-42.
Qiu J, Ozawa M, Terasaki PI. Liver transplantation in the United States. Clin Transpl 2005:17-28.
Radunz S, Juntermanns B, Kaiser GM et al. Efficacy and safety of linezolid in liver transplant patients. Transpl Infect Dis 2011;13:353-8.
Rahim MN, Long L, Penna L, Williamson C, et al. Pregnancy in Liver Transplantation. Liver Transpl. 2020 Apr;26(4):564-581.
Raimondo ML, Dagher L, Papatheodoridis GV, et al. Long-term mycophenolate mofetil monotherapy in combination with calcineurin inhibitors for chronic renal dysfunction after liver transplantation. Transplantation 2003;75:186-90. .
Reig M (b), Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022 Mar;76(3):681-693.
Ringe B, Wittekind C, Bechstein WO, et al. The role of liver transplantation in hepatobiliary malignancy. A retrospective analysis of 95 patients with particular regard to tumour stage and recurrence. Ann Surg 1989;209:88-98.
Roayie K, Feng S. Allocation policy for hepatocellular carcinoma in the MELD era: room for improvement? Liver Transpl 2007;13:S36-43.
Ridruejo E, Silva MO. Safety of long-term nucleos(t)ide treatment in chronic hepatitis B. Expert Opin Drug Saf 2012; 11: 357-60.
Rocha A, Lobato L. Liver transplantation in transthyretin amyloidosis: Characteristics and management related to kidney disease. Transplant Rev (Orlando) 2016; 31(2):115-120.
Rodríguez-Castro KI, De Martin E, Gambato M, Lazzaro S, Villa E, Burra P. Female gender in the setting of liver transplantation. World J Transplant 2014;4(4):229-42.
Rudraraju M, Osowo AT, Singh V, Carey EJ. Do patients need more frequent colonoscopic surveillance after liver transplantation? Transplant Proc 2008;40:1522-4.
Rupp C, Hippchen T, Bruckner T, et al. Effect of scheduled endoscopic dilatation of dominant strictures on outcome in patients with primary sclerosing cholangitis. Gut. 2019 Dec;68(12):2170-2178.
Saab S, Desai S, Tsaoi D, et al. Posttransplantation hepatitis B prophylaxis with combination oral nucleoside and nucleotide analogue therapy. Am J Transplant 2011;11:511-7.
Saglam K, Sahin TT, Ogut Z, et al. De Novo Malignancies After Liver Transplantation: Experience of a High-Volume Center. J Gastrointest Cancer. 2022 Dec;53(4):1020-1027.
Saidy RR, Sud I, Eurich F, et al. Discontinuation of Passive Immunisation Is Safe after Liver Transplantation for Combined HBV/HDV Infection. Viruses. 2021 May 13;13(5):904.
Saliba F (a), Dharancy S, Lorho R, et al. Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl 2011;17:905-13.
Saliba F (b), De Simone P, Nevens F et al. Efficacy and safety of everolimus with early reduction or elimination of tacrolimus in 719 de novo liver transplant recipients: 12 month results of a randomised, controlled study. Am J Transplant 2012;12(11):3008-20.
Sanchez EQ, Martin AP, Ikegami T, et al. Sirolimus conversion after liver transplantation: improvement in measured glomerular filtration rate after 2 years. Transplant Proc 2005;37:4416-23.
Saner FH, Cicinnati VR, Sotiropoulos G, Beckebaum S. Strategies to prevent or reduce acute and chronic kidney injury in liver transplantation. Liver Int 2012;32(2):179-88.
Satapathy SK, Joglekar K, Molnar MZ, et al. Achieving sustained virologic response in liver transplant recipients with hepatitis C decreases risk of decline in renal function. Liver Transpl 2018; doi: 10.1002/lt.25059. [Epub ahead of print].
Saunders EA, Engel B, Höfer A, et al. Outcome and safety of a surveillance biopsy guided personalised immunosuppression programme after liver transplantation. Am J Transplant. 2022 Feb;22(2):519-531.
Schiff E, Lai CL, Hadziyannis S, et al. Adefovir dipivoxil for wait-listed and post-liver transplantation patients with lamivudine-resistant hepatitis B: final long-term results. Liver Transpl 2007;13:349-60.
Schlitt HJ, Loss M, Scherer M, et al. Current developments in liver transplantation in Germany: MELD-based organ allocation and incentives for transplant centres. Z Gastroenterol 2011;49:30-8.
Schmeding M, Kiessling A, Neuhaus R et al. Mycophenolate mofetil monotherapy in liver transplantation: 5-year follow-up of a prospective randomised trial. Transplantation 2011;92:923-9.
Schnitzbauer AA, Sothmann J, Baier L, Bein T, Geissler EK, Scherer MN, Schlitt HJ. Calcineurin inhibitor free de novo immunosuppression in liver transplant recipients with pretransplant renal impairment: Results of a pilot study (PATRON07). Transplantation 2015;99(12):2565-75.
Schreiber PW, Bischoff-Ferrari HA, Boggian K, et al. Bone metabolism dynamics in the early posttransplant period following kidney and liver transplantation. PLoS One 2018;13(1):e0191167. doi: 10.1371/journal.pone.0191167. eCollection 2018.
Schulz MS, Gu W, Schnitzbauer AA, Trebicka J. Liver Transplantation as a Cornerstone Treatment for Acute-On-Chronic Liver Failure. Transpl Int. 2022 Mar 17;35:10108.
Shah JN, Haigh WG, Lee SP, Lucey MR, Brensinger CM, Kochman ML, Long WB, Olthoff K, Shaked A, Ginsberg GG. Biliary casts after orthotopic liver transplantation: clinical factors, treatment, biochemical analysis. Am J Gastroenterol 2003;98:1861–1867.
Sharma P, Schaubel DE, Messersmith EE, Guidinger MK, Merion RM. Factors that affect deceased donor liver transplantation rates in the United States in addition to the model for end-stage liver disease score. Liver Transpl. 2012;18:1456-63.
Shenoy A, Dienstag A, Dienstag P, et al. Scoring systems to assess relapse risk in alcohol use disorder presenting for early liver transplantation: A systematic review. Gen Hosp Psychiatry. 2021 Sep-Oct;72:23-30.
Shiraz AR, Nayeem MA, Agarwal S, Goyal N, Gupta S. Vascular complications in living donor liver transplantation at a high volume centre: Evolving protocols and trends observed over 10 years. Liver Transpl 2016; 23(4):457-64.
Shuster A, Huynh TJ, Rajan DK, Marquez MA, Grant DR, Huynh DC, Jaskolka JD. Response Evaluation Criteria in Solid Tumours (RECIST) criteria are superior to European Association for Study of the Liver (EASL) criteria at 1 month follow-up for predicting long-term survival in patients treated with transarterial chemoembolisation before liver transplantation for hepatocellular cancer.J Vasc Interv Radiol 2013;24(6):805-12.
Silva MF, Sherman M. Criteria for liver transplantation for HCC: What should the limits be? J Hepatol 2011;55:1137-47.
Silveira MG, Talwalkar JA, Lindor KD, Wiesner RH. Recurrent primary biliary cirrhosis after liver transplantation. Am J Transplant 2010;10:720-6.
Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH, ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol 2018; 113:175t94.
Singh S, Watt KD. Long-term Medical Management of the Liver Transplant Recipient: What the Primary Care Physician Needs to Know. Mayo Clin Proc 2012; 87(8): 779–790.
Smets F, Sokal EM. Epstein-Barr virus-related lymphoproliferation in children after liver transplant: role of immunity, diagnosis, and management. Pediatr Transplant 2002;6:280-7.
Soliman T, Hetz H, Burghuber C. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl 2007;13:1039-44.
Sotiropoulos GC, Malago M, Molmenti E, et al. Liver transplantation for hepatocellular carcinoma in cirrhosis: is clinical tumour classification before transplantation realistic? Transplantation 2005;79:483-7.
Sotiropoulos GC, Molmanti EP, Lösch C, Beckebaum S, Brolesch CE, Lang H. Meta-analysis of tumour recurrence after liver transplantation for hepatocellular carcinoma based on 1, 198 cases. Eur J Med Res 2007;12:527-34.
Sotiropoulos CG (a), Beckebaum S, Lang H, et al. Single-center experience on liver transplantation for hepatocellular carcinoma arising in alcoholic cirrhosis: results and ethical issues. Eur Surg Res 2008;40:7-13.
Sotiropoulos GC (b), Lang H, Sgourakis G, et al. Liberal policy in living donor liver transplantation for hepatocellular carcinoma: Lessons learned. Dig Dis Sci 2009;54(2):377-84.
Sotiropoulos GC, Molmenti EP, Lang H. Milan criteria, up-to-seven criteria, and the illusion of a rescue package for patients with liver cancer. Lancet Oncol 2009;10:207-8.
Sousa Da Silva RX, Weber A, Dutkowski P, Clavien PA. Machine perfusion in liver transplantation. Hepatology. 2022 Nov;76(5):1531-1549
Sposito C, Rossi RE, Monteleone M, et al. Postrecurrence Survival After Liver Transplantation for Liver Metastases From Neuroendocrine Tumors. Transplantation. 2021 Dec 1;105(12):2579-2586.
Staufer K, Andresen H, Vettorazzi E, et al. Urinary ethyl glucuronide as a novel screening tool in patients pre and post liver transplantation improves detection of alcohol consumption. Hepatology 2011;54(5):1640-9.
Sterneck M, Kaiser GM, Heyne N, et al. Everolimus and early calcineurin inhibitor withdrawal: 3-year results from a randomised trial in liver transplantation. Am J Transplant 2014;14(3):701-10.
Stirnimann G, Ebadi M, Czaja AJ, Montano-Loza AJ. Recurrent and de novo autoimmune hepatitis. Liver Transpl 2019;25(1):152-66.
Straś WA, Wasiak D, Łągiewska B, et al. Recurrence of Hepatocellular Carcinoma After Liver Transplantation: Risk Factors and Predictive Models. Ann Transplant. 2022 Jan 26;27:e934924.
Strassburg CP, Susanne Beckebaum, Andreas Geier et al. Practice guideline autoimmune liver diseases - AWMF-Reg. No. 021-27]. Z Gastroenterol 2017;55:1135-1226.
Stravitz TR, Shiffman ML, Kimmel M, et al. Substitution of tenofovir/emtricitabine for hepatitis B immune globulin prevents recurrence of Hepatitis B after liver transplantation. Liver Int 2012;32(7):1138-45.
Sugawara Y, Makuuchi M. Technical advances in living-related liver transplantation. J Hepatobiliary Pancreat Surg 1999;6:245-53.
Sugawara Y, Makuuchi M. Living donor liver transplantation: present status and recent advances. Br Med Bull 2005;75-76:15-28.
Suhling H, Gottlieb J, Bara C, et al. Chronic rejection: Differences and similarities in various solid organ transplants. Internist (Berl) 2016;57(1):25-37.
Sutcliffe RP, Maguire DD, Muiesan P, et al. Liver transplantation for Wilson’s disease: long-term results and quality-of-life assessment. Transplantation 2003;75:1003-6.
Sylvestre PB, Batts KP, Burgart LJ, Poterucha JJ, Wiesner RH. Recurrence of primary biliary cirrhosis after liver transplantation: Histologic estimate of incidence and natural history. Liver Transpl 2003;9:1086-93.
Tamura S, Sugawara Y, Kaneko J, Matsui Y, Togashi J, Makuuchi M. Recurrence of primary sclerosing cholangitis after living donor liver transplantation. Liver Int 2007;27:86–94.
Tanaka A, Kono H, Leung PSC, Eric Gershwin M. Recurrence of disease following organ transplantation in autoimmune liver disease and systemic lupus erythematosus. Cell Immunol 2020;347:104021.
Tang JT, Mao YM. Development of new drugs for the treatment of nonalcoholic steatohepatitis. J Dig Dis 2019. doi: 10.1111/1751-2980.12830.
Teefey SA, Hildeboldt CC, Dehdashti F, et al. Detection of primary hepatic malignancy in liver transplant candidates: prospective comparison of CT, MR imaging, US, and PET. Radiology 2003;226:533-42.
Teperman LW, Poordad F, Bzowej N, et al. Randomised trial of emtricitabine/tenofovir disoproxil fumarate after hepatitis B immunoglobulin withdrawal after liver transplantation. Liver Transpl 2013;19(6):594-601.
Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology 2018;67(4), 1560-99.
Testino G, Burra P, Bonino F, Piani F, et al.; Group of Italian Regions. Acute alcoholic hepatitis, end stage alcoholic liver disease and liver transplantation: an Italian position statement. World J Gastroenterol 2014;20(40):14642-51.
Thimonier E, Guillaud O, Walter T, et al. Conversion to everolimus dramatically improves the prognosis of de novo malignancies after liver transplantation for alcoholic liver disease. Clin Transplant 2014;28(12):1339-48.
Thursz M, Kamath PS, Mathurin P, Szabo G, Shah VH. Alcohol-related liver disease: Areas of consensus, unmet needs and opportunities for further studies. J Hepatol 2019;70:521-30.
Thursz M, Allison M. Liver transplantation for alcoholic hepatitis: Being consistent about where to set the bar. Liver Transpl 2018;24(6):733-734.
Tokodai K, Karadagi A, Kjaernet F, Romano A, Ericzon BG, Nowak G. Characteristics and risk factors for recurrence of nonalcoholic steatohepatitis following liver transplantation. Scand J Gastroenterol 2019;54(2):233-239.
Tokunaga S, Koda M, Matono T, et al. Assessment of ablative margin by MRI with ferucarbotran in radiofrequency ablation for liver cancer: comparison with enhanced CT. Br J Radiol 2012;85:745-52.
Toniutto P, Fabris C, Fumo E, et al. Pegylated versus standard interferon-alpha in antiviral regimens for posttransplant recurrent hepatitis C: comparison of tolerability and efficacy. J Gastroenterol Hepatol 2005;20:577-82.
Toso C, Meeberg GA, Bigam DL, et al. De novo sirolimus-based immunosuppression after liver transplantation for hepatocellular carcinoma: long-term outcomes and side effects. Transplantation 2007;83:1162-8.
Toso C, Mentha G, Kneteman NM, Majno P. The place of downstaging for hepatocellular carcinoma. J Hepatol 2010;52:930-6.
Tournoux-Facon C, Paoletti X, Barbare JC, et al. Development and validation of a new prognostic score of death for patients with hepatocellular carcinoma in palliative setting. J Hepatol 2011;54:108-14.
Tripathi D, Neuberger J. Autoimmune hepatitis and liver transplantation: indications, results, and management of recurrent disease. Semin Liver Dis 2009;29:286-96.
Trunecka P, Klempnauer J, Bechstein WO, Pirenne J, Friman S, Zhao A, Isoniemi H, Rostaing L, Settmacher U, Mönch C, Brown M, Undre N, Tisone G; DIAMOND study group. Renal function in de novo liver transplant recipients receiving different prolonged-release tacrolimus regimens-the DIAMOND Study. Am J Transplant 2015;15(7):1843-54.
Tung BY, Kimmey MB. Biliary complications of orthotopic liver transplantation. Dig Dis 1999;17:133-44.
Ulrich C, Degen A, Patel MJ, Stockfleth. Sunscreens in organ transplant patients. Nephrol Dial Transplant 2008;23:1805-8.
Vagefi PA, Ascher NL, Freise CE, Dodge JL, Roberts JP. The use of living donor liver transplantation varies with the availability of deceased donor liver transplantation. Liver Transpl 2012;18(2):160-5 .
Valentin N, Guerrido I, Rozenshteyn F, et al. Pregnancy Outcomes After Liver Transplantation: A Systematic Review and Meta-Analysis. Am J Gastroenterol. 2021 Mar 1;116(3):491-504.
Valentin-Gamazo C, Malago M, Karliova M, et al. Experience after the evaluation of 700 potential donors for living donor liver transplantation in a single center. Liver Transpl 2004; 10: 1087-96.
Vallejo GH, Romero CJ, de Vicente JC. Incidence and risk factors for cancer after liver transplantation.Crit Rev Oncol Hematol 2005;56:87-99.
van Rijn R, Schurink IJ, de Vries Y, et al.; DHOPE-DCD Trial Investigators. Hypothermic Machine Perfusion in Liver Transplantation - A Randomised Trial. N Engl J Med. 2021 Apr 15;384(15):1391-1401.
van Vugt JLA, Alferink LJM, Buettner S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol 2017;pii: S0168-8278(17)32474-1. doi: 10.1016/j.jhep.2017.11.030.
Vera A, Moledina S, Gunson B, et al. Risk factors for recurrence of primary sclerosing cholangitis of liver allograft. Lancet 2002;360:1943-4.
Verdonk RC, Buis CI, van der Jagt EJ, et al. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl 2007;13:725-32.
Victor DW, Monsour HP, Boktour M, et al. Outcomes of liver transplantation for hepatocellular carcinoma beyond the University of California San Francisco Criteria: A single-center experience. Transplantation 2020;104(1):113-121.
Vitale A, Bertacco A, Gambato M et al. Model for end-stage liver disease-sodium and survival benefit in liver transplantation. Transpl Int 2012;3.26(2):138-44.
Villeret F, Dharancy S, Erard D, et al. Inevitability of disease recurrence after liver transplantation for NAFLD cirrhosis. JHEP Rep. 2023 Jan 3;5(3):100668
Visseren T, Fuhler GM, Erler NS, et al. Recurrence of primary sclerosing cholangitis after liver transplantation is associated with specific changes in the gut microbiome pretransplant - a pilot study. Transpl Int. 2020 Nov;33(11):1424-1436.
Vogel A, Heinrich E, Bahr MJ, et al. Long-term outcome of liver transplantation for autoimmune hepatitis. Clin Transplant 2004;18:62-9.
Vogel A (b), Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022 Oct 15;400(10360):1345-1362.
Volpin R, Angeli P, Galioto A, et al. Comparison between two high-dose methylprednisolone schedules in the treatment of acute hepatic cellular rejection in liver transplant recipients: a controlled clinical trial. Liver Transpl. 2002 Jun;8(6):527-34
Washington K. Update on post-liver transplantation infections, malignancies, and surgical complications. Adv Anat Pathol 2005;12:221-6.
Weber NK, Forman LM, Trotter JF. HBIg discontinuation with maintenance oral anti-viral herapy and HBV vaccination in liver transplant recipients. Dig Dis Sci 2010;55:505-9.
Weiss KH, Gotthardt D, Schmidt J, et al. Liver transplantation for metabolic liver diseases in adults: indications and outcome. Nephrol Dial Transplant 2007;22, Suppl 8:viii9-12.
Weismüller TJ, Prokein J, Becker T, et al. Prediction of survival after liver transplantation by pretransplant parameters. Scand J Gastroenterol 2008;43:736-46.
Welling TH, Feng M, Wan S, et al. Neoadjuvant stereotactic body radiation therapy, capecitabine, and liver transplantation for unresectable hilar cholangiocarcinoma. Liver Transpl 2014;20(1):81-8.
Welzel TM, Reddy KR, Flamm SL et al. On-treatment HCV RNA in patients with varying degrees of fibrosis and cirrhosis in the SOLAR-
trial. Antivir Ther. 2016;21(6):541-546.
Wesdorp DJ, Knoester M, Coenraad MJ, et al. One-year results of tenofovir and emtricitabine without immunoglobulin to prevent hepatitis b recurrence after liver transplantation. J Hepatol 2012; 56 (Suppl. 2):S96-S97.
Wibaux C, Legroux-Gerot I, Dharancy S, et al. Assessing bone status in patients awaiting liver transplantation. Joint Bone Spine 2011; 78:387-91.
Wigg AJ, Gunson BK, Mutimer DJ. Outcomes following liver transplantation for seronegative acute liver failure: experience during a 12-year period with more than 100 patients. Liver Transpl 2005;11:27-34.
Wood NL, VanDerwerken D, Segev DL, Gentry SE. Correcting the sex disparity in MELD-Na. Am J Transplant. 2021 Oct;21(10):3296-3304
Wong PY, Portmann B, O’Grady JG, et al. Recurrence of primary biliary cirrhosis after liver transplantation following FK506-based immunosuppression. J Hepatol 1993;17:284-7.
Wong RJ, Aguilar M, Cheung R, Perumpail RB, Harrison SA, Younossi ZM, Ahmed A. Nonalcoholic steatohepatitis is the second leading aetiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology 2015;148(3):547-55.
Wu T, Morgan TR, Klein AS, Volk ML, Saab S, Sundaram V. Controversies in early liver transplantation for severe alcoholic hepatitis. Ann Hepatol 2018;17(5):759-68.
Yadav DK, Adhikari VP, Yadav RK, et al. Antiviral prophylaxis or preemptive therapy for cytomegalovirus after liver transplantation?: A systematic review and meta-analysis. Front Immunol. 2022 Nov 10;13:953210.
Yahyazadeh A, Beckebaum S, Cicinnati V, et al. Efficacy and safety of subcutaneous human HBV-immunoglobulin (Zutectra) in liver transplantation: an open, prospective, single-arm phase III study. Transpl Int 2011;24:441-50.
Yang Y, Zhao JC, Yan LN, et al. Risk factors associated with early and late HAT after adult liver transplantation. World J Gastroenterol. 2014;20(30):10545-52.
Yao FY, Xiao L, Bass NM, Kerlan R, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: validation of the UCSFexpanded criteria based on preoperative imaging. Am J Transpl 2007;7:2587-96.
Yoo MC, Vanatta JM, Modanlou KA, et al. Steroid-free liver transplantation using rabbit antithymocyte globulin induction in 500 consecutive patients. Transplantation 2015;99(6):1231-5.
Yoshida H, Kato T, Levi DM, et al. Lamivudine monoprophylaxis for liver transplant recipients with non-replicating hepatitis B virus infection. Clin Transplant 2007;21:166-71.
Yoshida EM, Marotta PJ, Greig PD, et al. Evaluation of renal function in liver transplant recipients receiving daclizumab (Zenapax), mycophenolate mofetil, and a delayed, low-dose tacrolimus regimen vs. a standard-dose tacrolimus and mycophenolate mofetil regimen:
a multicenter randomised clinical trial. Liver Transpl 2005;11:1064-72.
Younossi ZM, Blissett D, Blissett R, et al. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology 2016;64(5):1577-86.
Younossi ZM, Ratziu V, Loomba R, Rinella M, Anstee QM, Goodman Z, Bedossa P, Geier A, Beckebaum S, et al; REGENERATE Study Investigators. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394(10215):2184-2196.
Yu L, Ioannou GN. Survival of liver transplant recipients with haemochromatosis in the United States. Gastroenterology 2007;133:489-95.
Zaffar N, Soete E, Gandhi S, Sayyar P, Van Mieghem T, 2, D’Souza R. Pregnancy outcomes following single and repeat liver transplantation: An international two-centre cohort. Liver Transpl 2018. doi: 10.1002/lt.25071. [Epub ahead of print].
Zavaglia C, De Carlis L, Alberti AB, et al. Predictors of long term survival after liver transplantation for hepatocellular carcinoma. Am J Gastroenterol 2005;100:2708-16.
Zhu J, Hussaini T, Yoshida EM. Early liver transplant for severe alcoholic hepatitis: establishing a new frontier by ignoring the rule? Ann Transl Med 2018;6(20):411.
Zimmerman MA, Trotter JF, Wachs M, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl 2008;14:633-8.
Zimmermann T, Beckebaum S, Berg C, et al. Expert recommendations: Hepatitis C and transplantation]. Z Gastroenterol 2016;54(7):665-84.
Zoepf T, Maldonado-Lopez EJ, Hilgard P, et al. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl 2006;12:88-94.
Zoepf T, Maldonado de Dechêne EJ, Dechêne A, et al. Optimised endoscopic treatment of ischemic-type biliary lesions after liver transplantation. Gastrointest Endosc 2012;76:556-63.
