18. Diagnosis, prognosis & therapy of hepatocellular carcinoma
Ursula Ehmer, Jens U. Marquardt
Summary
Hepatocellular carcinoma (HCC) is one of the most common and deadliest cancers worldwide. Historically, viral hepatitis and alcohol abuse constitute dominant risk factors of HCC development. However, non-alcoholic fatty liver disease is a rapidly evolving cause of HCC in the Western world. In cirrhotic patients, diagnosis of HCC can be reliably established by dynamic imaging modalities. However, the relevance of histology becomes increasingly recognised due to improved precision medicine approaches. A variety of treatment options is now available, and treatments depend on the stage of disease as well as the degree of liver function impairment. However, despite established surveillance by ultrasound, the majority of cases are still diagnosed at advanced tumour stages when treatment options are limited. Curative treatment approaches include liver transplantation, surgical resection, percutaneous ablation, and radiation, whereas different local and systemic therapies are available in advanced stages. Thus, HCC is a hallmark for multidisciplinary dialogue in tumour boards. Further, the landscape of systemic therapies significantly evolved with the advent of targeted therapies and immune checkpoint inhibitors over the recent years. Currently, combination therapies are the gold standard for upfront therapy in eligible patients at advanced stages of the disease and steadily improved overall survival over the last decade. Nevertheless, prognosis of HCC patients is still limited and there remains an urgent need for novel diagnostic and predictive biomarkers as well as improved therapies.
Epidemiology, Screening and Prevention
Liver cancer ranks among the most common cancers and is the third most frequent cause of cancer-related death worldwide (Vogel 2022). Hepatocellular carcinomas (HCC) represent about 90% of primary liver cancers and show a significant increase in all age populations over the last decades. Globally, primary liver cancer accounts for around 7% of all cancers and affected more than 905 000 patients in 2020 (Ferlay 2021). Further, mortality rates equal incidence rates and, thus, HCC advanced to a major global health care problem. Notably, HCC is characterised by a significant geographic heterogeneity that is associated with incidence rates of the major risk factors. Worldwide, the most frequent underlying etiologies are chronic viral hepatitis (B and C), alcohol abuse, aflatoxin as well as inherited or acquired metabolic diseases, including haemochromatosis, alpha-1-antitrypsin deficiency as well as non-alcoholic steatohepatitis (NASH). The latter showing the most prominent increase in incidence rates in Western countries due to a sharp rise in metabolic syndrome, obesity, and diabetes mellitus type 2.
In the majority of HCC cases, advanced liver fibrosis or cirrhosis can be detected and, thus, the presence of liver cirrhosis remains the most important risk factor for the development of HCC. Overall, annual incidence among patients with cirrhosis is 1-8%, depending on the underlying etiological risk factor. In addition, co-existing risk factors as well as other patient-related factors resembling age, male gender as well as the degree of portal hypertension aggravate the risk for liver cancer development. Interestingly, although a significant number of non-alcoholic fatty liver disease (NAFLD)/NASH patients also show overlapping alcohol abuse, HCC in the background of metabolic inflammation can be induced in the absence of cirrhosis in a sizeable number of patients, which underlines the increasing importance of metabolic liver diseases in the Western world.
Most important preventive measure in the context of chronic liver diseases (CLDs) is early detection as well as prevention of cirrhosis development. Besides vaccination and treatment in chronic Hepatitis B, consequent treatment of HCV as well as elimination of noxes are particularly important. Notably, the role of screening for CLDs in the general population remains a matter of ongoing discussion and should be addressed in global health care programmes (Labenz 2022). Besides the mentioned measures, daily coffee consumption seems to have beneficial effects in CLD. In addition, metformin shows positive effects on the development of HCC in patients at risk but should only be given in case of a medical indication, i.e., diabetes mellitus type 2.
Surveillance of patients at high risk
According to general recommendations, surveillance should be performed in patients at high risk for HCC development with a high probability of curative treatment options (Voesch 2022). In general, an annual incidence of 1.5% warrants surveillance in cirrhotic patients irrespective of the aetiology and, thus, the majority of patients with liver cirrhosis should be enrolled in specific surveillance programmes when liver function is still compensated, i.e. CHILD A/B or CHILD C on the waiting list for transplantation. In Caucasian patients with HBV, risk assessment can be reliably achieved by implementing the PAGE-B score. Surveillance should be installed for patients with intermediate or high risk, i.e. PAGE-B score >10 (Papatheodoridis 2016). Surveillance in the absence of cirrhosis should be reserved to patients with an age <50 years and chronic HBV infection in patients of African and Asian descent. Notably, the relevance of surveillance in non-cirrhotic NASH and HCV patients remains unclear and is a matter of scientific interest. However, in case of suspected advanced fibrosis, an increased risk for HCC development is documented and regular surveillance seems warranted.
Surveillance is generally recommended by means of bi-annual abdominal ultrasound and should be performed by experienced personnel. The use of other dynamic imaging technologies including computer tomography or MRI have a high false positive rate and, thus, does not seem to be cost effective for the majority of patients. However, if ultrasound is not feasible due to patient factors, e.g., obesity or abdominal gas, contrast enhanced dynamic imaging can be considered. Serological markers including repeated AFP measurements can optionally be used to complement ultrasound, but the overall diagnostic sensitivity remains poor. Thus, novel biomarkers for early detection are urgently needed and are the focus of ongoing studies.
Diagnosis of hepatocellular carcinoma
HCC differs from most other tumour entities as it can be reliably diagnosed based on specific characteristics by MRI or CT imaging in cirrhotic patients. Nevertheless, in non-cirrhotic patients or whenever diagnostic criteria for HCC are not fulfilled by imaging, diagnosis should be confirmed by biopsy. As the interventional risks in obtaining liver biopsies are small, some centres aim to secure diagnosis by histopathology in all palliative cases also when radiologic characteristics confirm the presence of HCC (European Association for the Study of the Liver 2018). However, increased risk of bleeding after liver biopsy should be considered in cirrhotic patients with severely impaired plasmatic coagulation or low platelets as well as in patients with clinically meaningful perihepatic ascites. Another concern is needle-track seeding of tumour cells, which is reported to occur in less than 3% of patients. Seeding metastasis can be treated by resection or radiation therapy in most cases (Silva 2008). Most importantly, there seems to be no influence on the oncologic outcome or overall survival. Therefore, histological confirmation is desired and should not be restricted to unclear situations (Fuks 2014).
Clinical presentation
Early and even intermediate stage HCC are mostly asymptomatic. Liver nodules at these stages are often detected by surveillance ultrasound in patients at risk, by routine medical check-up, or during imaging for other medical conditions. At more advanced tumour stages, patients can present with tumour-specific symptoms such as pain, weight loss and fatigue as well as worsening of liver function and other cirrhosis-related symptoms – mostly ascites or variceal bleeding – due to increased portal pressure or macrovascular invasion into the main portal vein. More rarely, intraabdominal hemorrhage from rupture of subcapsular liver tumours leads to the diagnosis of HCC (Sahu 2019). Consequently, diagnostic work-up of a worsening of liver function in cirrhotic patients – including de-novo ascites – should always rule out an underlying HCC.
Imaging-based diagnosis
With the prominent role of imaging in the diagnosis and staging of HCC, refined algorithms for radiologic work-up of liver nodules in the patients with cirrhosis have been developed. Specific changes in vascularisation are observed during HCC development – i.e. hypervascularisation in the (late) arterial phase together with a wash-out in the portal venous and/or delayed venous phases – and are the backbone of imaging-based HCC diagnosis. Multi-phase contrast-enhanced imaging methods can detect these chances with high sensitivity and specificity in nodules ≥ 1 cm. MRI is currently considered the most sensitive imaging method (Di Martino 2013). Sensitivity increases with size of the tumour nodule, ranging between 62% and 71% (MRI), or 62% and 68% (CT), respectively, for small nodules < 20 mm and up to 95% (MRI) or 92% (CT) in larger nodules (Aube 2017, Lee 2015). Apart from “classic” imaging features, several other criteria can be helpful to establish the diagnosis of HCC. In MRI, consideration of diffusion-weighted imaging (DWI) and the use hepatobiliary contrast agents increase the specificity of diagnosis. In addition to classical arterial hyperenhancement and portal venous or delayed phase washout, the Liver Imaging Reporting and Data System (LI-RADS) criteria for HCC diagnosis include further features such as enhancing capsule appearance, size, threshold growth by ≥50% in ≤6 months, and restricted diffusion to categorise lesions in cirrhotic patients. LI-RADS categories reflect the likelihood of any nodule for malignancy and specifically for HCC (Lee 2021). The LI-RADS criteria also consider that sensitivity and specificity of radiologic imaging will likely never reach 100% and even “probably benign” lesions (LI-RADS 2) have a probability of HCC in 1 out of 10 patients. Therefore, biopsies should be performed in all doubtful cases to avoid any delay in treatment. Contrast-enhanced ultrasound (CE-US) may help to establish the diagnosis of HCC but is considered less sensitive and – especially in differentiation from cholangiocarcinoma – less specific than radiologic imaging (Piscaglia 2017). However, due to its low cost and easy application, CE-US remains a relevant diagnostic tool in many centres, but it should not be used as sole imaging method in the diagnosis of HCC.
In small liver lesions below 1 cm, sensitivity and specificity of imaging-based diagnosis remains low and sampling for histopathology can provide a technical challenge. In these cases, HCC diagnosis cannot be reliably established. With a high risk of progression of these nodules towards unambiguous HCC in cirrhotic patients, follow-up by imaging every three months is strongly recommended (Khalili 2011). Of note, all imaging features of HCC should only be used for diagnosis in cirrhotic patients and in patients at high risk for HCC – such as patients with chronic HBV – due to the high pre-test probability in these cohorts. In all other cases, diagnosis should be confirmed by biopsy even if imaging is highly suggestive of HCC.
Whenever HCC diagnosis is confirmed either by imaging or histopathology, a complete tumour staging including CT scan of the lung and abdomen – if not already covered by diagnostic MRI of the liver – should be obtained to rule out metastatic disease.
Due to the unique vascular pattern of intrahepatic HCC, assessment of treatment response should not only consider changes in tumour size, but also changes in vascularisation pattern. Especially in intra-arterial treatment approaches (TACE or TARE), loss of arterial (hyper-)enhancement is considered a criterion for treatment response. This feature is included in modified Response Evaluation Criteria in Solid Tumors (mRECIST) for HCC (Llovet 2020). Further adaption of the mRECIST criteria might be needed to account for specific changes observed with immunotherapeutic treatment approaches to accurately describe tumour response.
Histology and biomarkers
Histological Classification of malignant liver tumours is the basis for subsequent diagnostic and therapeutic approaches. By histology, several specific subtypes of HCC have been identified. The revised WHO classification distinguishes eight specific subtypes found in up to 35% of HCC (steatohepatitic, clear cell, macrotrabecular-massive, scirrhous, chromophobe, fibrolamellar, neutrophil-rich, and lymphocyte-rich), while the remaining tumours (approx. 65%) are classified as “not-otherwise-specified” HCC (NOS-HCC). Some subtypes are associated with distinct genetic changes and characterised by a better or worse prognosis in comparison to NOS-HCC. However, subtyping currently does not affect clinical decision making (Lokuhetty).
A diagnostic challenge – particularly in early HCC – remains the distinction between dysplastic nodules and well-differentiated HCC. In these cases, an immunohistochemistry panel consisting of glypican 3, HSP70 and glutamine synthetase can confirm malignant tumour growth with high specificity and a sensitivity of 70% (Di Tommaso 2009). Therapeutically relevant is the differentiation of HCC from other malignant liver tumours. In samples where differentiation of HCC and intrahepatic cholangiocarcinoma (iCCA) is not possible by histomorphology, immunohistochemistry of cell-type specific markers such as HepPar-1 and arginase 1 (hepatocytes) or CK7 and CK19 (bile duct cells) can be used to establish diagnosis. Tumours with biliary differentiation components in addition to the hepatocellular differentiation should be delineated as combined HCC/(i)CCA. Histology is also crucial in the differentiation of highly differentiated HCC from precursor lesions, i.e., dysplastic nodules, as well as non-malignant hepatocellular adenoma and focal nodular hyperplasia.
Though elevated AFP levels are suggestive of HCC, a relatively low sensitivity of 60% for AFP levels >20 ng/mL renders it unsuitable as a sole marker for early detection. However, APF levels >100 ng/mL are highly specific for HCC (98%) and might help to establish diagnosis in unclear cases where biopsy is deemed too risky for the patient. Several serologic biomarkers for early diagnosis of HCC as well as for monitoring of therapeutic response are currently under investigation, including AFP-L3, DCP or neutrophil/lymphocyte ratio. However, no reliable markers have been established in clinical routine so far. Additionally, the concept of “liquid biopsy” – using blood samples to detect circulating tumour cells, extracellular vesicles, and circulating tumour DNA (ctDNA) – has gained more attention in recent years. While still far from clinical application, this approach might give future opportunities for early detection of HCC and provide diagnostic tools for estimating prognosis and therapeutic response (Pinero 2020).
Classification of HCC
Clinical staging of HCC aims to stratify patients with respect to specific prognosis and to select the optimal therapeutic options for the respective stage. Herein, the Barcelona Clinic Liver Cancer (BCLC) classification has been adopted as the international standard, which is recommended by both the American Association for the Study of Liver Diseases (AASLD) and the European Association for the Study of the Liver (EASL) (Table 1) (European Association for the Study of the Liver 2018). Unlike other classification schemes the BCLC staging system does not exclusively rely on tumour characteristic and spread, but also includes performance status as well as severity of liver disease (Llovet 1999). Importantly, the classification also provides information on median survival of patients as well as recommendations for specific therapeutic options (Table 1). Importantly, given the increase in therapeutic options in advanced stages of HCC, a current update included a clinical decision-making tool for the recommendation that considers individual patients preferences as well as co-morbidities (Reig 2022). Despite intense research activities, molecular characteristics are not yet able to reliably assess individual prognosis or response prediction of patients with HCC.
Table 1. BCLC Staging System| BCLC Stage | ECOG | Tumour Characteristics | Child-Pugh Stage | Prognosis |
| 0 | 0 | Single <2cm | A | >5 years |
| A | 0 | Single <5cm or ≤3 nodules <3cm | A | >5 years |
| B | 0 | Multinodular | A | >2.5 years |
| C | 1-2 | Macrovascular invasion, extrahepatic spread | A | >2 years |
| D | 3-4 | any | B9 – C | 3 months |
Notably, the BCLC classification might be less accurate in Asian patients with a distinct etiological background. An alternative classification, the Hong Kong Liver Cancer Staging System (HKLC), has been recently introduced and might be more accurate in predicting survival of affected Asian patients (Yau 2014). Several other classification schemes to predict prognosis of patients have been introduced over the recent years. Particularly relevant for both prognosis as well as stage-dependent response to therapy is the so-called ALBI score that combines serum albumin and bilirubin. Validity of the score could be confirmed in geographically distinct cohorts of patients as well as different disease stages (Johnson 2015).
Treatment allocation according to the BCLC staging system
The BCLC staging systems provides guidance for the choice of treatment in HCC patients (Figure 1) as outlined in more detail below. However, many patients do benefit from treatment strategies that do not strongly adhere to the staging system. More specifically, curative treatment options might not be available for all patients with very early or early HCC due to tumour or patient characteristics. In these cases, locoregional treatment options can be more suitable and offer good tumour control. Likewise, in patients with intermediate stage HCC (BCLC B) vascular anatomy might preclude the use of intraarterial therapies and justify systemic treatment, as does insufficient response to locoregional treatment approaches. On the other hand, superior response to systemic or locoregional treatments might deem tumours confined to the liver resectable and therefore amenable for curation in BCLC B patients. The adaptation of BCLC treatment recommendations to individual tumour characteristics have been recognised as the concept of “stage migration” and highlight the importance of an individualised therapy tailored to each HCC patient (Reig 2022).
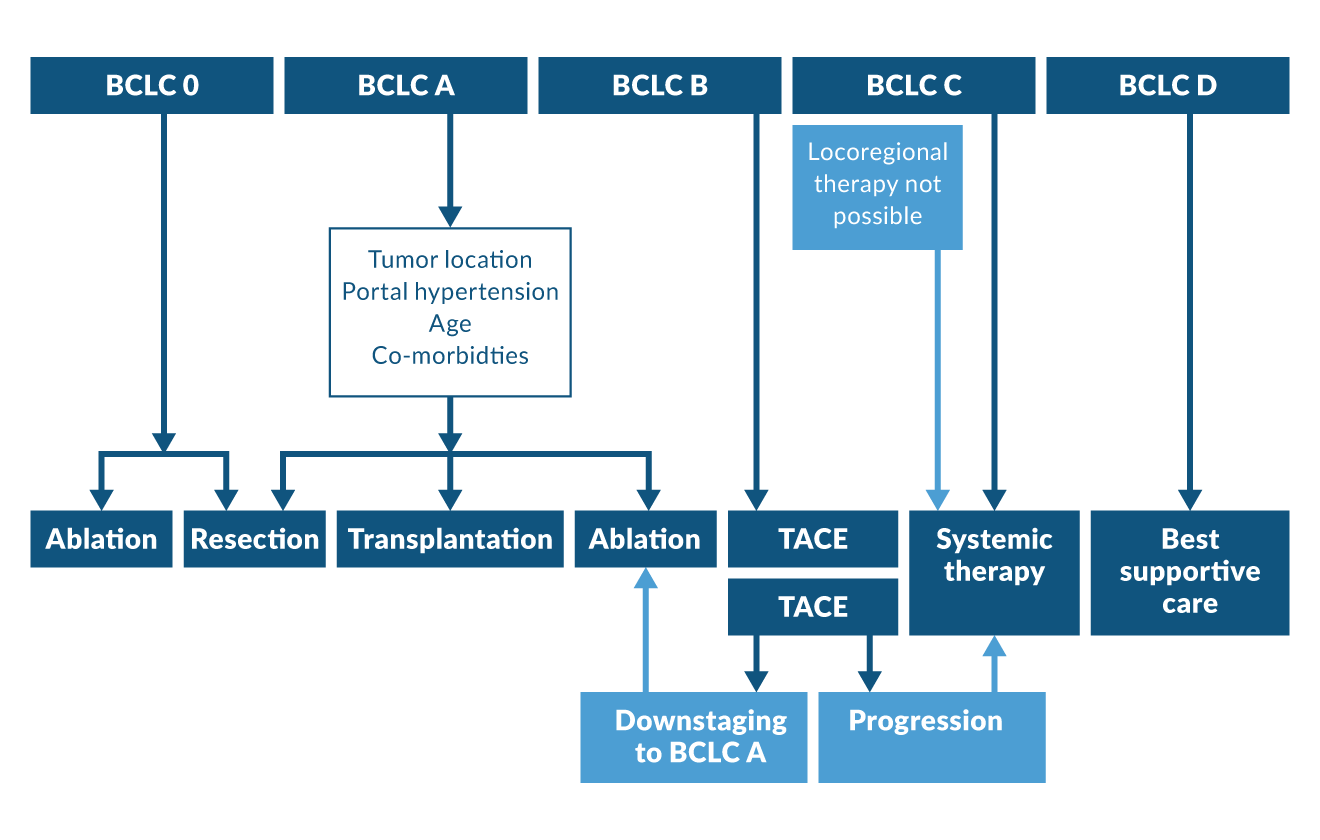 Figure 1. BCLC treatment algorithm for HCC
Figure 1. BCLC treatment algorithm for HCC
Curative treatment approach in BCLC stages 0-A
Established curative treatment approaches in HCC include surgical resection, liver transplantation and local ablation of tumour nodules. The choice of treatment depends on multiple factors that include size and location to the tumour, presence of multifocal lesions, liver function and liver functional reserve, presence of portal hypertension, age, performance status, and any medical preconditions that might influence the therapeutic outcome for a certain procedure.
Surgical resection
In BCLC stages 0 and A, resection is the therapy of choice as long as complete removal of the tumour(s) is possible and save for the patient, i.e., liver function is preserved. Additionally, tumours beyond Milan criteria can be evaluated for surgery if there is no evidence of metastasis or macrovascular invasion. Especially in non-cirrhotic HCC patients, extensive resections are possible due to the comparatively large functional reserve of the remaining liver (Zhou 2014). In general, tumour growth beyond the liver and the presence of extrahepatic metastases are strong indicators of a poor prognosis and high recurrence rates. Therefore, patients with these more advanced tumours should not be considered for surgery.
Recent advances in locoregional and systemic therapies have resulted in an increasing percentage of patients with an excellent tumour response – rendering previously unresectable tumours suitable for potentially curative surgery in many cases. The term conversion therapy has been established for this relatively novel treatment strategy in HCC. However, the benefits of this approach are still considered as controversial since reliable outcome predictors are lacking (Sun 2021). Especially in more advanced tumour stages such as those tumours with macrovascular invasion, further studies are needed to identify tumour characteristics that can predict which patients will benefit from conversion therapy.
Reduced functional liver reserve after resection remains the main predictor of peri-operative mortality in HCC patients with liver cirrhosis. The extend of resection that is feasible and save for the patient depends on liver function and the presence of portal hypertension. Reduced platelets (< 100, 000/µl), increased liver stiffness (> 12-14 kPa), and mildly impaired liver function (MELD ≥ 9 and/or reduced hepatic indocyanine green kinetics (ICG test)) are non-invasive predictors of an increased risk for post-operative hepatic decompensation or even liver failure. Similarly, the presence of oesophageal varices or an increased hepatic venous pressure gradient (HVPG > 10 mmHg) is associated with an unfavourable outcome after surgery. In these patients, only minor liver resections of <3 segments should be performed (Citterio 2016). In patients with a more severely impaired liver function (Child-Pugh B or above), even small surgical resections cannot be considered as save and are associated with a high mortality.
Despite these challenges, the boundaries set by liver cirrhosis have been pushed towards more extensive surgeries in the recent years. Minimal-invasive resections are associated with lower complication and mortality rates in comparison to open resections while displaying comparable recurrence and survival rates (Andreou 2018). Thus, minimal-invasive approaches should be implemented whenever feasible. In addition, preserving liver function remains a key prognostic factor in surgery in HCC patients. Extra-anatomic versus anatomic resections save liver parenchyma but might be associated with a higher rate of tumour recurrence as part of the tumour-bearing portal region remains in situ (Jiao 2020). Additionally, techniques to increase the functional reserve of the future liver remnant have been successfully used in patients with and without liver cirrhosis. Among those, pre-operative portal vein embolisation and associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) are established techniques. However, due to the high mortality rate of up to 30%, these approaches should be only a considered in selected patients (Allaire 2020).
Even though surgical resection is considered as a curative treatment approach, recurrence rates are high with a global 5-year recurrence-free survival of only approx. 35% and a limited overall survival of less than 60%, respectively (Reveron-Thornton 2022). Tumour recurrence rates are associated with larger size and number of tumour nodules as well as poor differentiation, the presence of microvascular invasion, and high alpha-fetoprotein levels. Current guidelines recommend follow-up of HCC patients after surgery as most recurrent tumours are amenable to treatment (European Association for the Study of the Liver 2018). Most HCC recurrences are intrahepatic, and many centres implement CT or MRI imaging every 3 – 6 months after resection for 2 years or longer, though recommendations for follow-up vary between guidelines.
Liver transplantation
Liver transplantation should be considered in all patients with unresectable HCC within Milan criteria (BCLC A) as long as there are no contraindications. Even in cirrhotic patients after curative surgery, liver transplantation might be considered due to the high rate of tumour recurrence. The option for liver transplantation is implemented into most current guidelines as the treatment of choice in early unresectable HCC. However, many patients will not be considered for transplantation due to advanced age or the presence of relevant co-morbidities. While advanced age is not considered as contra-indication for liver transplantation, the overall fitness or “biological age” is still relevant to predict post-operative mortality. However, with a peak HCC incidence at approximately 70 years of age (El-Serag 2011), probability of concomitant cardiovascular disease or secondary malignancies is relevant in this cohort, resulting to the exclusion of many patients from liver transplantation. The rising incidence of HCC in non-alcoholic fatty liver disease is also associated with a higher rate of cardiovascular diseases, diabetes, and severe obesity that can increase the risk of post-operative complications and result in poor long-term outcomes. As a shortage of donor organs presents a challenge in many countries, candidates for liver transplantation are carefully selected and a tight control of any risk factors is mandatory in patients considered for liver transplantation.
Despite these limitations, tumour-related long-term outcome after liver transplantation is excellent, generally exceeding 80% 5-year survival rate, and recurrence rates are low for patients within the Milan criteria (BCLC A) (Mazzaferro 1996). While the size limits of Milan or United Network of Organ Sharing (UNOS) T2 criteria, respectively, have been implemented into transplant guidelines more than 20 years ago, it is now acknowledged that patients with larger tumours and a higher number of tumour nodules will have a comparable outcome to BCLC A patients if tumours meet specific criteria. There are a number of “extended criteria” that focus on identifying patients with tumours beyond Milan criteria but with a low risk of recurrence, most prominently the UCSF criteria (single tumour ≤ 6.5 cm or no more than 3 tumours with the largest one not exceeding 4.5 cm and a combined tumour diameter of no more than 8 cm) and up-to-7 criteria (single tumour ≤ 7 cm or multiple tumours with the sum of the diameter of the larges tumour and the number of tumours ≤ 7). Both lead to an excellent 5-year survival rate of more than 70% after liver transplantation (Mazzaferro 2009, Yao 2001). On the other hand, even patients within Milan criteria might have a high risk of recurrence if they have high AFP levels. Currently, AFP levels > 1, 000 ng/mL (persisting after downstaging) are considered as a contraindication for liver transplantation and some extended criteria include AFP levels into their calculation (Duvoux 2012, Mazzaferro 2018). Even with the development more refined extended criteria, liver transplantation is not considered for patients with macrovascular invasion or even metastasis due an unfavourable cancer biology and poor prognosis after transplantation (Roayaie 2004).
Independent of the initial tumour extent, long waiting times for a donor organ due to organ shortage present a relevant risk for tumour progression in HCC patients (Bhoori 2010). To minimise this risk, locoregional “bridging” therapies such a transarterial chemoembolisation (TACE), radioembolisation, ablation, or stereotactic body radiation (SBRT) are used to prevent tumour progression. Importantly, response to these bridging therapies can be used as a predictor for outcome as good responders are characterised by low recurrence rates after transplant (Beal 2016, Rubinstein 2017). Down-staging of tumours to meat Milan criteria has also been implemented into transplant allocation systems in several countries as outcomes in these patients are comparable to those who were always with Milan criteria (Marrero 2018, Yao 2015).
Even with optimal patient selection, tumour recurrence after transplantation presents an eminent risk. With the lack of adjuvant therapies in HCC suitable for transplant patients, the choice of immunosuppression has been studied as an influencing factor for tumour recurrence. Inhibitors of mTOR such as sirolimus and everolimus have anti-tumour as wells as immunosuppressive properties. Though no significant survival benefit could be shown in a large randomised controlled trial for the treatment with sirolimus-containing combination therapy (Geissler 2016), several retrospective studies indicate that HCC patients benefit from the use of mTOR inhibitors in combination with reduced calcineurin inhibitors in their immunosuppressive regimen (Yan 2022). Due to considerable side effects such as thrombosis of the hepatic artery and impaired wound healing, mTOR inhibitors should not be started earlier than one month after liver transplantation.
With limited evidence for standardised follow-up imaging for tumour recurrence, sonography as well as CT or MRI might be used for up to 5 years after transplant depending on the individual recurrence risk based on explant histology.
Ablation
Thermal ablation is an alternative treatment approach with curative intent that is considered equal to surgical resection in smaller tumours of up to 2 cm (Wang 2014). However, ablation leads to lower recurrence-free and overall survival rates in larger tumours (Shin 2021, Uhlig 2019). During procedures using thermal ablation, a probe is inserted into the tumour under CT or ultrasound guidance with subsequent destruction of tumour tissue by heat. Radiofrequency ablation (RFA) and microwave ablation (MWA) are both thermo-ablative techniques with comparable outcome. Recurrence from tumour margins and a cooling effect from adjacent large blood vessels that are believed to counteract ablation – commonly called the “heat sink” phenomenon – both likely contribute to inferior outcome of ablation in comparison to surgical resection in tumours larger than 15 mm (Kang 2018). Additionally, tumour location is important and not all tumours are eligible for ablation: tumour nodules close to the hilus or to heat-sensitive organs such as the gall bladder or colon are not ideal candidates for ablation. However, treatment in most subcapsular nodules is safe and efficient (Kang 2016).
As ablation seems to be well tolerated even in cirrhotic patients or patients with a high perioperative risk profile, this approach can also be considered in in larger tumours up to 5 cm in cases where the risk of surgery is high. In addition to an adequate safety margin in ablation, a combination of TACE and ablation improves recurrence-free and overall survival in lesions up to 7 cm in comparison to RFA alone (Peng 2013).
Other techniques that are less commonly used and are often technically challenging are cryoablation, irreversible electroporation (IRE), laser induced thermal therapy (LITT), and high-intensity focused ultrasound (HIFU). Though data from controlled trials is limited, efficiency might be comparable to RFA and MWA in smaller tumours (Qian 2021). In centres experienced in these techniques, they can present alternative treatment strategies when thermal ablation is not possible.
Adjuvant therapy
Until recently, there was no sufficient evidence for a benefit of an adjuvant therapy after curative resection. Prophylactic TACE after resection that targets the resection margins is primarily applied in some centres in China, but only selected patients seem to benefit from this approach and there no data from controlled studies so far (Wang 2021). The randomised controlled phase 3 STORM-trial showed no benefit of adjuvant Sorafenib in a large patient cohort after curative ablation or resection (Bruix 2015). The first phase 3 trial in adjuvant treatment of HCC that met its primary endpoint is the IMbrave 050 trial that investigated an adjuvant combination therapy with atezolizumab and bevacizumab in HCC patients with high-risk of recurrence after ablation or resection (Qin 2023). In this study, post-operative or post-interventional treatment with atezolizumab and bevacizumab in patients with high-risk tumours (tumour size >5 cm, more than 3 tumours, presence of microvascular invasion or limited macrovascular invasion, and/or poor tumour differentiation) significantly improved recurrence-free survival in comparison to placebo. In addition, results from several trials investigating adjuvant immunotherapy are currently pending. Importantly, adjuvant immunotherapies should strictly be avoided after liver transplantation due to the high risk of fatal rejection.
Locoregional therapies in BCLC stages B and C
For large or multinodular tumours that cannot be treated by surgery, several locoregional therapies, which can achieve long-term disease control, are used for palliative treatment. In BCLC stage B, improvements in locoregional and systemic therapies have led to an increase in survival to > 2.5 years (Reig 2022). In selected cases such as limited macrovascular invasion in tumours confined to the liver, patients with BCLC C stage HCC can also benefit from locoregional therapies. However, with the availability of highly efficient systemic therapies, locoregional treatments are now less commonly used in advanced HCC.
TACE and DEB-TACE
TACE is considered the gold standard in the treatment of intermediate stage HCC. Several trials report a survival benefit in comparison to symptomatic treatment (Llovet 2002). Though treatment protocols are poorly standardised, a combination of chemotherapy and embolising agent delivered through transarterial catheter into the liver presents the cornerstone of all TACE procedures. Chemotherapeutic agents commonly used in conventional TACE – or cTACE – are doxorubicin, epirubicin or cisplatin which are injected in an emulsion containing lipiodol, an iodised oil with embolising properties (Lencioni 2016), and can be combined with other embolising agents. As an alternative approach, TACE can also be performed with drug-eluting beads (DEB-TACE), where chemotherapeutic agents are bound to embolic microspheres and slowly released into the tumour microenvironment. While initially developed to reduce systemic exposure to chemotherapeutic agents, several studies now indicate that clinical outcomes of cTACE and DEB-TACE are comparable (Gao 2013).
Unselective TACE is associated with a higher rate of side effects – most prominently a decrease in liver function and post-embolisation syndrome with fever and abdominal pain. Thus, selective and supra-selective TACE are now the standard of care. Treatment should be applied as selectively as possible to target tumour-feeding vessels while protecting surrounding liver tissue from ischemic injury (European Association for the Study of the Liver 2018). TACE is most effective in limited multinodular disease. Depending on the size of the tumour, TACE treatment can be repeated several times with the goal of complete devascularisation of the tumour. In patients with insufficient response to TACE – defined by tumour growth despite TACE or occurrence of multiple new intrahepatic lesions indicative of rapid tumour progression – treatment should be discontinued and a switch to systemic therapies is recommended.
Transarterial Radioembolisation (TARE)
TARE or selective internal radiotherapy (SIRT) presents an alternative intraarterial treatment with results comparable to TACE in intermediate stage HCC (Brown 2022, Kolligs 2015). A more recent phase II trial even showed improved time to progression and overall survival in patients treated with TARE in comparison to those treated with DEB-TACE (Dhondt 2022). However, TARE should not be used in advanced HCC, since several large trials showed no improvement in overall survival compared to systemic treatment with sorafenib and the emerging systemic treatment modalities in this stage (Chow 2018, Vilgrain 2017). Radioembolisation is usually performed using glass or resin microspheres loaded with yttrium-90 (90Y). Microspheres are delivered via the hepatic artery and emit high-energy beta-particles with a half-life of 64 hours. For adequate dosimetry and to exclude relevant misplacement of microspheres into non-tumour tissue within and outside the liver, angiographic evaluation using 99mTc macro-aggregated albumin (99mTc-MAA) is performed prior to TARE. A more recent development is the use of Holmium-166 (166Ho) coated microspheres. 166Ho is a beta-emitting radionuclide which also emits gamma photons – a characteristic that allows the use of the 166Ho microspheres for dosimetry at lower doses (Weber 2022). Similar to TACE, radioembolisation aims to target tumour nodules as selectively as possible, sparing non-tumourous liver tissue while applying high and if achievable ablative doses of the radionuclide to the tumour. In selected cases – especially when resection or ablation are not possible in smaller tumours – delivery of ablative doses to the tumour bearing segment can be used with a curative intent (SIRT segmentectomy). Treatment of a large volume of non-tumourous liver should be avoided as it poses a higher risk of radiation-induces liver disease (REILD) and of a long-term decrease in liver function. However, if one or more tumour nodules are restricted to one liver lobe, delivery of high-radiation doses can induce hypertrophy of the untreated lobe with a latency of several months. This approach can be used for downstaging may enable resection of the tumour in selected patients (Salem 2023).
Stereotactic body radiation therapy (SBRT)
Radiation therapies take advantage of the comparatively high radiation sensitivity of hepatocellular carcinoma. SBRT allows for focal delivery of high radiation doses to individual tumours. Due to the lack of randomised controlled trials, SBRT is not recommended as a fist line therapy in most guidelines. However, it can present an alternative treatment option if ablation is not possible. SBRT has shown excellent local control rates of >90% and an overall survival >70% after 3 years in smaller HCC <6cm in a meta-analysis of several observational studies (Long 2021) and seems to be relatively well tolerated in patients with impaired liver function (Feng 2018). Additionally, SBRT can be used as bridging to liver transplant in cases where tumours are not amenable for or do not respond to TACE or ablation. In addition to SBRT, other radiation-based therapies are currently under investigation such as brachytherapy and proton beam therapy, which are available at selected centres.
Systemic therapy in BCLC stage C
Historic view on systemic treatment options – the era of multi-tyrosine kinase inhibitors
Sorafenib – The gold standard in first-line therapy for more than 10 years
Until 2007, no effective treatments for patients diagnosed with advanced HCC or patients who progressed to this stage after failure of other therapies were available. The positive results of the randomised, controlled phase III SHARP (Sorafenib Hepatocellular Carcinoma Assessment Randomised Protocol) trial evaluating sorafenib, an oral multi-tyrosine kinase inhibitor (TKI) with activity against vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and RAF kinase for advanced HCC in a mainly Western cohort provided first evidence for the efficacy of anti-angiogenetic strategies in advanced HCC (Llovet 2008). Median OS in the sorafenib arm was 10.7 months versus 7.9 months in placebo-treated patients (hazard ratio [HR] 0.69; 95% CI 0.55–0.87; p = 0.00058). Similar results were not only demonstrated in a parallel phase III study involving mainly Asian, predominantly hepatitis B-infected patients, but also in eight subsequent phase III studies in which sorafenib served as the control treatment (Cheng 2009, Llovet 2021). On the basis of the positive results from both trials, sorafenib was approved and became the systemic standard of care across different therapeutic lines. Importantly, none of the following phase III trials could demonstrate superiority over sorafenib until recently. Although currently no predictive biomarkers for response exist, several clinical factors including chronic hepatitis C infection or side effects including early dermatological events or hypertension favour a better response to the treatment (Bruix 2019). Despite approval for all stages of liver disease, large non-interventional observational studies have shown that the survival of patients with CHILD class B cirrhosis is significantly shorter than those of patients with CHILD A cirrhosis. Since these studies did not provide conclusive evidence for a benefit in patients with severely impaired liver function, the use of sorafenib should in general be limited to patients with compensated stages of cirrhosis.
Lenvatinib – REFLECT Trial
Lenvatinib is another oral multi-tyrosine kinase inhibitor with activity against VEGFR1–3, fibroblast growth factor receptor (FGFR) 1–4, PDGF, RET and KIT. An open-label phase III study involving mainly Asian patients was conducted to demonstrate non-inferiority of lenvatinib in comparison with sorafenib in a first-line setting (Kudo 2018). The study reached its primary endpoint with a median OS of 13.6 months in the experimental lenvatinib arm versus 12.3 months in the sorafenib arm (HR 0.92; 95% CI 0.79–1.06). An interesting observation of this trial was the high objective response rate (ORR) for lenvatinib with 24.1% versus 9.2% for sorafenib despite the similar OS. Further, surrogate characteristics for survival such as progression-free survival (PFS) and time to progression (TTP) were consistently higher in the lenvatinib arm than in the sorafenib arm (PFS: 7.4 months vs 3.7 months; TTP: 8.9 months vs 3.7 months). Adverse effects were overall slightly more pronounced in lenvatinib-treated patients, particularly hypertension and thrombocytopenia. Importantly, the study excluded patients with adverse prognostic tumour characteristics such as main branch portal vein thrombosis or tumours involving > 50% of the liver. Nevertheless, results from the trial encouraged the use of lenvatinib as an effective first-line alternative in advanced HCC, leading to its inclusion in recent EASL and European Society for Medical Oncology (ESMO) guidelines.
Compounds in first-line treatment with no therapeutic benefits in phase 3 trials
Following the approval of sorafenib, several other first-line substances have been tested either against sorafenib (brivanib, linifanib, sunitinib) or in combination with sorafenib (sorafenib plus erlotinib, sorafenib plus doxorubicin). Despite positive signals from phase II trials, none of the studies achieved their primary endpoint and demonstrated a meaningful survival benefit over sorafenib alone.
A new era of therapies – first-line immunotherapy with checkpoint inhibitor monotherapy and combination therapy
The recent advances in immune-oncological therapies spiked great hopes for their efficacy for treatment of HCC patients. In the first large phase III study, the so-called Checkmate-459 study, monotherapy with nivolumab (anti-PD-1 antibody) was tested in comparison to sorafenib in patients with advanced HCC in first-line therapy (Yau 2022). Although an improved survival of 16.4 months versus 14.7 months was achieved, the study did not reach statistical significance and missed its primary endpoint (hazard ratio 0.85 [95% confidence interval (CI) 0.72-1.02]; p=0.075). Based on the results of the trial, it was reasonable to assume that monotherapy with a checkpoint inhibitor might not be sufficiently effective in HCC. Accordingly, subsequent studies targeted combination of immune-oncology (IO) therapy with different partners. Combinations of dual immunotherapy combining PD-1/PD-L1 and CTLA4, immunotherapy with anti-VEGF antibodies or TKIs are currently investigated (Heinrich 2018). The recently concluded IMbrave 150 trial (Finn 2020) investigated the combination of the PD-L1 antibody atezolizumab (Atezo) and the VEGF antibody bevacizumab (Bev). Treatment with the new combination resulted in an overall survival of 19.2 versus 13.2 months with sorafenib (HR 0.58) and prolonged progression-free survival (6.8 months with Atezo/Bev vs. 4.3 months with sorafenib; HR 0.59). Following FDA and, subsequently, EMA approval in November 2020, the combination with Atezo/Bev is now the new standard of care in first-line systemic therapy for eligible patients with advanced HCC (Figure 2). A key advantage of the new combination therapy is that it is usually very well tolerated in clinical practice and maintains patients' quality of life for a long time. However, the risk of bleeding, especially fulminant bleeding from oesophageal varices, represents a serious clinical challenge. Thus, a thorough screening should be obligatorily before therapy initiation. Of note, a necessary variceal ligation prior to therapy initiation may delay the start of systemic therapy and potentially cause tumour progression. Hence, lenvatinib and sorafenib remain important alternative treatment options in non-eligible patients, i.e., following liver transplantation or uncontrolled autoimmune disease (Figure 2).
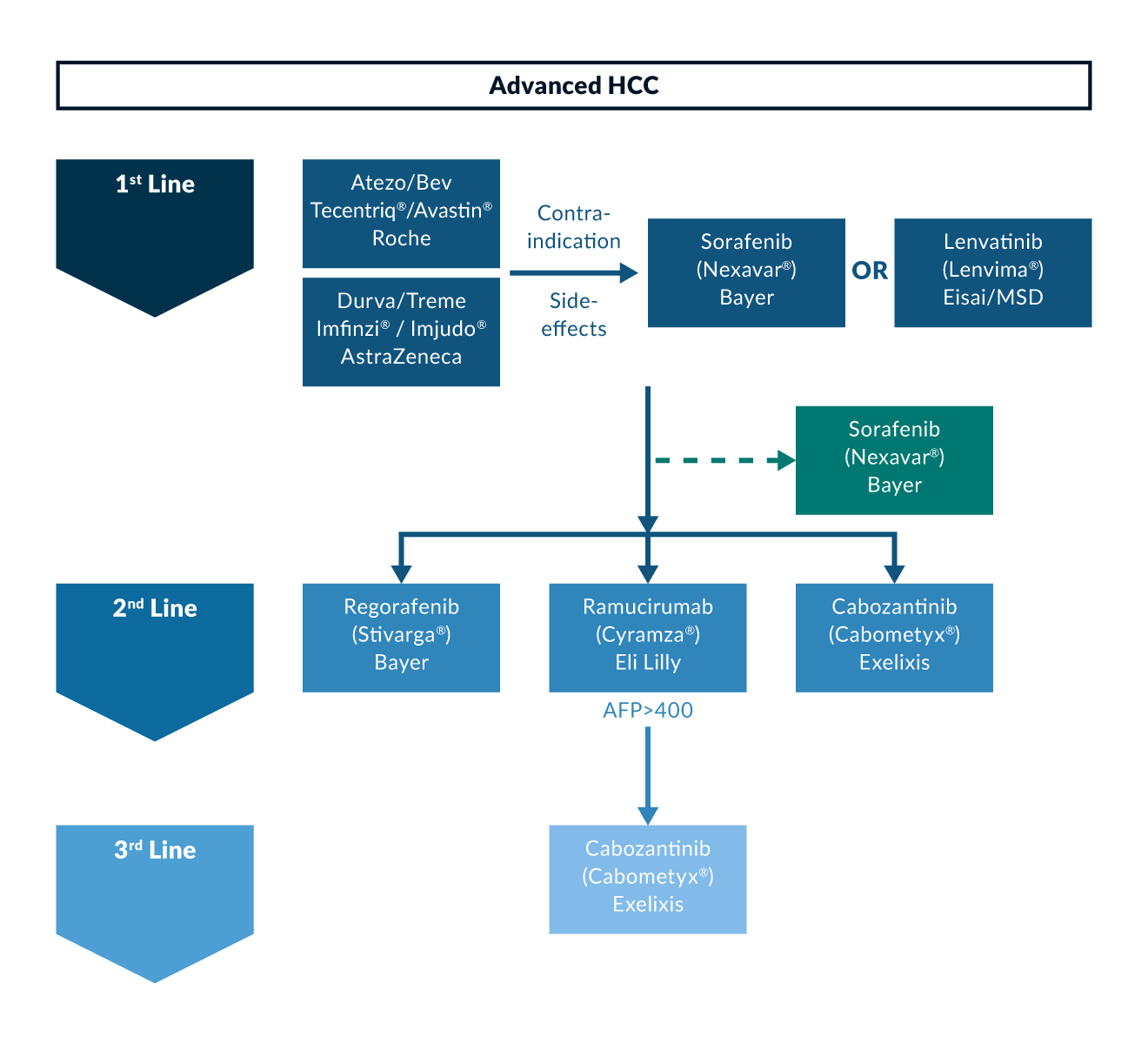 Figure 2. Systemic treatment lines in advanced HCC
Figure 2. Systemic treatment lines in advanced HCC
In the recent phase III HIMALAYA trial, both combination therapy of the PD-L1 inhibitor durvalumab (anti-PD-L1 antibody) in combination with a single dose of tremelimumab (antibody against cytotoxic T-lymphocyte-associated protein 4) were investigated in the so-called STRIDE protocol (Single Tremelimumab Regular Interval Durvalumab) in first-line treatment of advanced HCC as well as durvalumab monotherapy against a comparator arm with the previous standard of care sorafenib. Initial results of the trial were presented at the ASCO-GI Congress in January 2022 (https://evidence.nejm.org/doi/full/10.1056/EVIDoa2100070). Median overall survival was 16.43 months (95% CI, 14.16 – 19.58) with STRIDE, 16.56 months (95% CI, 14.06 – 19.12) with durvalumab, and 13.77 months (95% CI, 12.25 – 16.13) with sorafenib. The hazard ratio for STRIDE versus sorafenib was 0.78 (p = 0.0035) and survival with durvalumab mono was non-inferior to therapy with sorafenib (HR 0.86). In terms of adverse events, combination therapy also showed a significant improvement in patients' quality of life compared with sorafenib. The combination was approved by the FDA in the United States in October 2022, followed by EMA approval in the EU in February 2023. The results of the Himalaya study, thus, underscore the efficacy of IO combination therapy for HCC.
Besides the combination of anti-VEGF and PD-1/PD-L1 inhibitor and immunotherapy combinations, other strategies involve the combination of TKI and PD-1/PD-L1 inhibitors. The role of the combination is currently unclear and has to be demonstrated in future clinical phase III trials (Llovet 2022).
Second-Line Therapies
Regorafenib – RESORCE Trial
Regorafenib is an oral TKI, that is structurally a fluorinated sorafenib analogue with a similar spectrum of molecular targets. Besides a profound anti-proliferative effect on the tumour cells, regorafenib significantly inhibits neo-angiogenesis and, thus, modulates the tumour microenvironment. The randomised controlled RESORCE phase III trial evaluated the role of regorafenib in patients with advanced HCC that progressed under sorafenib therapy (Bruix 2017). The main inclusion criteria were a preserved liver function (CHILD A), progressive disease under sorafenib as well as tolerability to sorafenib (defined as receiving sorafenib ≥ 400 mg for at least 20 days of the last 28 days of treatment). The study further rigorously stratified for region, portal-vein thrombosis, alpha-fetoprotein (AFP) levels and extrahepatic tumour manifestation. This highly selective strategy was performed to avoid toxicity and unequal distribution of prognostically adverse characteristics. The study reached its primary endpoint and demonstrated a significantly improved OS for regorafenib (10.6 months) versus placebo (7.8 months) (HR 0.63; 95% CI 0.50–0.79; p < 0.0001) as well as an increase in the median TTP (3.2 months vs 1.5 months; HR 0.44; 95% CI 0.36–0.55; p < 0.001). In addition, regorafenib significantly extended the tumour control rates as well as ORR. The spectrum of adverse events was comparable to side effects described for sorafenib, including hypertension, hand-foot syndrome, fatigue, and diarrhoea, but were overall manageable. Based on the results of the RESORCE trial, regorafenib was approved by the FDA and the EMA in patients with advanced HCCs previously treated with sorafenib. Notably, a retrospective evaluation of the sequential treatment effect of sorafenib followed by regorafenib revealed a median OS from the beginning of the systemic therapy of 26 months versus 19.6 months for placebo (Finn 2018). These data obtained in a well selected patient population provided, for the first time, evidence that sequential application of systemic therapies in Barcelona Clinic Liver Cancer-stage C (BCLC C) patients can reach comparable survival times observed in phase III trials of TACE in BCLC-B patients. Thus, a sequential treatment strategy should be prospectively implemented and evaluated in suitable patients (Marquardt 2019).
Cabozantinib – CELESTIAL Trial
Cabozantinib is another oral multi-tyrosine kinase inhibitor with activity against MET, VEGFR2, and RET. Following its approval for the treatment of thyroid and renal cell carcinomas by both the EMA and the FDA, cabozantinib has most recently been granted approval as a second-line treatment in HCC Child–Pugh A patients by the EMA and the FDA (Abou-Alfa 2018). The phase III CELESTIAL trial compared the benefit of cabozantinib (60 mg daily) with placebo in second- and third-line treatment for advanced HCC with preserved liver function and good performance status (i.e., Child–Pugh A, Eastern Cooperative Oncology Group (ECOG) performance status (PS) 0/1). The study was stopped after the second interim analysis due to proven efficacy. Overall, an improvement in OS from 8.0 months to 10.2 months could be demonstrated for cabozantinib compared with placebo. Mean PFS was 5.4 months versus 1.9 months (HR 0.44; 95% CI 0.36–0.52; p < 0.001). Further, the disease control rate was 64% for cabozantinib versus 33.4% in placebo (p < 0.001) with a low ORR rate of 4% versus 0.4% according to RECIST 1.1 (p = 0.0086). Similar to the other TKIs, grade 3/4 side effects occurred in 68% of patients and predominantly involved hand-foot syndrome (17 vs 0%), hypertension (12 vs 2%), transaminase elevation (12 vs 7%), and fatigue (10 vs 4%). Interestingly, nearly 30% of patients in the study had received more than one pre-treatment, albeit most of these patients had been treated with chemotherapy in addition to sorafenib. Nevertheless, the results of the CELESTIAL study suggest that cabozantinib could also have a place in later therapy lines (Figure 2). Interestingly, a recent analysis confirmed the efficacy of cabozantinib over placebo in patients with different AFP levels, but most prominently in patients with AFP levels ≥ 400 ng/mL, which determines a poor prognosis subgroup of patients. In this cohort, the median OS was 8.5 months compared with 5.2 months with cabozantinib or placebo, respectively (HR 0.71; 95% CI 0.54–0.94) [21].
Ramucirumab – REACH-2
Ramucirumab is a recombinant monoclonal antibody that specifically binds to the VEGFR2 domain, thereby preventing the binding of VEGF ligands. Similar to other compounds, such as sunitinib and brivanib, ramucirumab initially showed promising results in a small phase II study for advanced HCC (Nault 2018). Based on these results, the randomised controlled phase III REACH study was initiated as a second-line therapy after sorafenib failure (Zhu 2015). However, the REACH study failed to demonstrate a significant improvement in median OS for all patients and did not meet its primary endpoint. Despite these initial discouraging results, a subgroup analysis suggested that ramucirumab improves survival in patients with elevated baseline AFP levels above 400 ng/mL. Subsequently, the REACH II study was initiated in this patient population (Zhu 2019). In this selected cohort, ramucirumab improved the median OS from 7.3 months to 8.5 months versus placebo (HR 0.710; 95% CI 0.53–0.95; p = 0.019) and PFS from 1.6 months to 2.8 months (HR 0.452; 95% CI 0.40–0.60; p < 0.0001). A combined analysis of the REACH I and II study confirmed the survival benefit of ramucirumab compared with placebo (Delta: 3.1 months; HR 0.69; 95% CI 0.57–0.84; p = 0.0002). Thus, ramucirumab is an interesting second-line option in patients with high AFP levels and a poor prognosis. Notably, ramucirumab is the first intravenous, non-TKI drug with proven anti-angiogenetic efficacy in second line for advanced HCC. Accordingly, the side-effect spectrum deviates substantially from multi-tyrosine kinase inhibitors. With respect to grade 3/4 side effects, only hypertension (12.7% vs 3.8%) and proteinuria (1.3% vs 0%) occurred more frequently with ramucirumab compared with placebo.
Several other compounds were evaluated against placebo in second-line settings for advanced HCC. Neither brivanib, everolimus nor tivantinib showed a significant improvement in OS.
Second-line checkpoint inhibitor monotherapy and combination therapy.
Initial evidence on the efficacy of checkpoint inhibition with pembrolizumab in the second-line setting after failure or intolerance of lenvatinib were revealed by the KEYNOTE-224 trial (Zhu 2018). Building on the results of this trial, the phase III KEYNOTE-240 trial was initiated (Finn 2019). Despite a significantly improved OS (13.9 months for pembrolizumab compared with 10.6 months for placebo (HR: 0.781; 95% CI: 0.611 to 0.998; p = 0.0238), the study did not reach the prespecified significance level and is, therefore, formally negative, despite showing comparable benefit to the phase II study and clear clinical benefit in terms of durable response in patients who responded to treatment.
The single-arm phase 1/2 CheckMate 040 study evaluated the combination of nivolumab and ipilimumab (El-Khoueiry 2017). The study included patients previously treated with sorafenib for advanced HCC were randomised to 3 treatment arms: arm 1: nivolumab 1 mg/kg and ipilimumab 3 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg every 2 weeks; arm 2: nivolumab 3 mg/kg and ipilimumab 1 mg/kg every 3 weeks (4 doses), followed by nivolumab 240 mg every 2 weeks; and arm 3: nivolumab 3 mg/kg every 2 weeks and ipilimumab 1 mg/kg every 6 weeks. The primary endpoints were safety and tolerability, and secondary endpoints included ORR, DOR, DCR, and OS. In arm 1, ORR was 31%, with 7 patients achieving complete tumour response; OS was 23 months. The combination was well tolerated, with 37% treatment-related grade 3/4 adverse events (mostly pruritus and rash). Based on the results of this phase 1/2 study, the nivolumab-ipilimumab combination received accelerated approval from the FDA, but has not received approval from the EMA.
| Agent | Type | Line | FDA | EMA |
| Nivolumab | Anti-PD-1 | 2L | 09/2017 | – |
| Pembrolizumab | Anti-PD-1 | 2L | 11/2018 | – |
| Atezolizumab (in combination with Bevacizumab) | Anti-PD-L1 | 1L | 05/2020 | 11/2020 |
| Ipilimumab (in combination with Nivolumab) | Anti-CTLA-4 | 2L | 3/2020 | – |
| Durvalumab (in combination with Tremelimumab) | Anti-PD-L1 | 1L | 10/2022 | 02/2023 |
Supportive therapy in end-stage liver disease – BCLC stage D
Maintaining liver function is the key dogma in HCC and constitutes the most significant prognostic factor. Irrespective of the treatment modality, clinical outcomes are undoubtfully better in patients with preserved liver function. Thus, any treatment that can result in a decrease in liver function, such as unselective TACE or TARE, might – even if temporally tolerated – diminish long-term outcome. In systemic therapy with sorafenib, Child-Pugh B patients have a poorer outcome compared to patients with preserved liver function (Child-Pugh A) and are more likely to discontinue treatment due to side effects (Marrero 2016). If these findings hold true in combination immunotherapies that are better tolerated than sorafenib remains to be investigated. More recent evidence indicates that systemic therapy with atezolizumab and bevacizumab is reasonably tolerated in Child-Pugh B patients and there is currently no rational to withhold treatment from this subset of patients (D'Alessio 2022).
Nevertheless, treatment of liver cancer should be mostly restricted to patients with preserved liver function. For this reason, terminal stage HCC (BCLC D) is not defined by tumour size or extension, but rather by presence of severely impaired liver function (Child-Pugh C). In this subset of patients, survival is believed to depend on liver function und is estimated to be shorter than 3 months. Therefore, these patients should only receive supportive therapy as any tumour-directed treatments will not result in a survival benefit but, conversely, reduce liver function even further. As a more accurate predictor of liver functional reserve in HCC patients, the ALBI score – which is calculated from albumin and bilirubin levels – has been developed. ALBI grade 3 corresponds to an impaired liver function and as in Child-Pugh C patients, no benefit from tumour-specific therapies can be expected (Pinato 2017). Needless to say, an exception from this rule are patients awaiting liver transplantation. In those patients, control of the tumour is more important than a decrease in liver function as long as the patient remains fit for transplant.
Key points
- The majority of HCC develop in cirrhotic or fibrotic livers – with alcohol abuse, chronic viral hepatitis, and non-alcoholic fatty liver disease (NAFLD) presenting the main risk factors for cirrhosis and HCC development.
- HCC diagnosis in cirrhotic livers can be based on characteristic imaging criteria, but histological confirmation of HCC is recommended in palliative cases.
- The BCLC treatment algorithm is the backbone of stage-adapted HCC therapy in the Western world.
- Curative treatment options in very early (BCLC 0) and early (BCLC A) HCC include resection, ablation, and liver transplantation.
- Locoregional treatment approaches including transarterial chemoembolisation (TACE) and transarterial radioembolisation (TARE) are used in multifocal HCC confined to the liver (intermediate stage HCC, BCLC B)
- In advanced stage HCC – characterised by macrovascular tumour invasion and/or metastatic spread (BCLC C) – systemic combination immunotherapies present the standard of care in first line treatment, while different tyrosine kinase inhibitors are available as alternative or second line therapeutic options.
- All tumour-directed therapies should be restricted to patients with preserved liver function.
References
Abou-Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379(1):54-63.
Allaire M, Goumard C, Lim C, Le Cleach A, Wagner M, Scatton O. New frontiers in liver resection for hepatocellular carcinoma. JHEP Rep. 2020;2(4):100134.
Andreou A, Struecker B, Raschzok N, et al. Minimal-invasive versus open hepatectomy for hepatocellular carcinoma: Comparison of postoperative outcomes and long-term survivals using propensity score matching analysis. Surg Oncol. 2018;27(4):751-758.
Aube C, Oberti F, Lonjon J, et al. EASL and AASLD recommendations for the diagnosis of HCC to the test of daily practice. Liver Int. 2017;37(10):1515-1525.
Beal EW, Dittmar KM, Hanje AJ, et al. Pretransplant Locoregional Therapy for Hepatocellular Carcinoma: Evaluation of Explant Pathology and Overall Survival. Front Oncol. 2016;6:143.
Bhoori S, Sposito C, Germini A, Coppa J, Mazzaferro V. The challenges of liver transplantation for hepatocellular carcinoma on cirrhosis. Transpl Int. 2010;23(7):712-722.
Brown AM, Kassab I, Massani M, et al. TACE versus TARE for patients with hepatocellular carcinoma: Overall and individual patient level meta analysis. Cancer Med. 2022.
Bruix J, da Fonseca LG, Reig M. Insights into the success and failure of systemic therapy for hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2019;16(10):617-630.
Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
Bruix J, Takayama T, Mazzaferro V, et al. Adjuvant sorafenib for hepatocellular carcinoma after resection or ablation (STORM): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2015;16(13):1344-1354.
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34.
Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective Internal Radiation Therapy Versus Sorafenib in Asia-Pacific Patients With Hepatocellular Carcinoma. J Clin Oncol. 2018;36(19):1913-1921.
Citterio D, Facciorusso A, Sposito C, Rota R, Bhoori S, Mazzaferro V. Hierarchic Interaction of Factors Associated With Liver Decompensation After Resection for Hepatocellular Carcinoma. JAMA Surg. 2016;151(9):846-853.
D'Alessio A, Fulgenzi CAM, Nishida N, et al. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology. 2022;76(4):1000-1012.
Dhondt E, Lambert B, Hermie L, et al. (90)Y Radioembolisation versus Drug-eluting Bead Chemoembolisation for Unresectable Hepatocellular Carcinoma: Results from the TRACE Phase II Randomised Controlled Trial. Radiology. 2022;303(3):699-710.
Di Martino M, De Filippis G, De Santis A, et al. Hepatocellular carcinoma in cirrhotic patients: prospective comparison of US, CT and MR imaging. Eur Radiol. 2013;23(4):887-896.
Di Tommaso L, Destro A, Seok JY, et al. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50(4):746-754.
Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including alpha-fetoprotein improves the performance of Milan criteria. Gastroenterology. 2012;143(4):986-994 e983; quiz e914-985.
El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017.
El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365(12):1118-1127.
European Association for the Study of the Liver E. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182-236.
Feng M, Suresh K, Schipper MJ, et al. Individualised Adaptive Stereotactic Body Radiotherapy for Liver Tumors in Patients at High Risk for Liver Damage: A Phase 2 Clinical Trial. JAMA Oncol. 2018;4(1):40-47.
Ferlay J, Colombet M, Soerjomataram I, et al. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021.
Finn RS, Merle P, Granito A, et al. Outcomes of sequential treatment with sorafenib followed by regorafenib for HCC: Additional analyses from the phase III RESORCE trial. J Hepatol. 2018;69(2):353-358.
Finn RS, Qin S, Ikeda M, et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382(20):1894-1905.
Finn RS, Ryoo BY, Merle P, et al. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomised, Double-Blind, Phase III Trial. J Clin Oncol. 2019:JCO1901307.
Fuks D, Cauchy F, Fusco G, Paradis V, Durand F, Belghiti J. Preoperative tumour biopsy does not affect the oncologic course of patients with transplantable HCC. J Hepatol. 2014;61(3):589-593.
Gao S, Yang Z, Zheng Z, et al. Doxorubicin-eluting bead versus conventional TACE for unresectable hepatocellular carcinoma: a meta-analysis. Hepatogastroenterology. 2013;60(124):813-820.
Geissler EK, Schnitzbauer AA, Zulke C, et al. Sirolimus Use in Liver Transplant Recipients With Hepatocellular Carcinoma: A Randomised, Multicenter, Open-Label Phase 3 Trial. Transplantation. 2016;100(1):116-125.
Heinrich B, Czauderna C, Marquardt JU. Immunotherapy of Hepatocellular Carcinoma. Oncol Res Treat. 2018;41(5):292-297.
Jiao S, Li G, Zhang D, Xu Y, Liu J, Li G. Anatomic versus non-anatomic resection for hepatocellular carcinoma, do we have an answer? A meta-analysis. Int J Surg. 2020;80:243-255.
Johnson PJ, Berhane S, Kagebayashi C, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. J Clin Oncol. 2015;33(6):550-558.
Kang TW, Lim HK, Cha DI. Percutaneous ablation for perivascular hepatocellular carcinoma: Refining the current status based on emerging evidence and future perspectives. World J Gastroenterol. 2018;24(47):5331-5337.
Kang TW, Lim HK, Lee MW, et al. Long-term Therapeutic Outcomes of Radiofrequency Ablation for Subcapsular versus Nonsubcapsular Hepatocellular Carcinoma: A Propensity Score Matched Study. Radiology. 2016;280(1):300-312.
Khalili K, Kim TK, Jang HJ, et al. Optimisation of imaging diagnosis of 1-2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilisation. J Hepatol. 2011;54(4):723-728.
Kolligs FT, Bilbao JI, Jakobs T, et al. Pilot randomised trial of selective internal radiation therapy vs. chemoembolisation in unresectable hepatocellular carcinoma. Liver Int. 2015;35(6):1715-1721.
Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173.
Labenz C, Arslanow A, Nguyen-Tat M, et al. Structured Early detection of Asymptomatic Liver Cirrhosis: Results of the population-based liver screening programme SEAL. J Hepatol. 2022;77(3):695-701.
Lee YJ, Lee JM, Lee JS, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015;275(1):97-109.
Lee YT, Wang JJ, Zhu Y, Agopian VG, Tseng HR, Yang JD. Diagnostic Criteria and LI-RADS for Hepatocellular Carcinoma. Clin Liver Dis (Hoboken). 2021;17(6):409-413.
Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolisation for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64(1):106-116.
Llovet JM, Bru C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19(3):329-338.
Llovet JM, Castet F, Heikenwalder M, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19(3):151-172.
Llovet JM, Kelley RK, Villanueva A, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2021;7(1):6.
Llovet JM, Lencioni R. mRECIST for HCC: Performance and novel refinements. J Hepatol. 2020;72(2):288-306.
Llovet JM, Real MI, Montana X, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359(9319):1734-1739.
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
Lokuhetty D, White VA, Watanabe R, Cree IA, World Health Organisation., International Agency for Research on Cancer. Digestive system tumours. Fifth edition. ed.
Long Y, Liang Y, Li S, et al. Therapeutic outcome and related predictors of stereotactic body radiotherapy for small liver-confined HCC: a systematic review and meta-analysis of observational studies. Radiat Oncol. 2021;16(1):68.
Marquardt JU, Saborowski A, Czauderna C, Vogel A. The Changing Landscape of Systemic Treatment of Advanced Hepatocellular Carcinoma: New Targeted Agents and Immunotherapies. Target Oncol. 2019;14(2):115-123.
Marrero JA, Kudo M, Venook AP, et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J Hepatol. 2016;65(6):1140-1147.
Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68(2):723-750.
Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10(1):35-43.
Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693-699.
Mazzaferro V, Sposito C, Zhou J, et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology. 2018;154(1):128-139.
Nault JC, Galle PR, Marquardt JU. The role of molecular enrichment on future therapies in hepatocellular carcinoma. J Hepatol. 2018;69(1):237-247.
Papatheodoridis G, Dalekos G, Sypsa V, et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J Hepatol. 2016;64(4):800-806.
Peng ZW, Zhang YJ, Chen MS, et al. Radiofrequency ablation with or without transcatheter arterial chemoembolisation in the treatment of hepatocellular carcinoma: a prospective randomised trial. J Clin Oncol. 2013;31(4):426-432.
Pinato DJ, Sharma R, Allara E, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. J Hepatol. 2017;66(2):338-346.
Pinero F, Dirchwolf M, Pessoa MG. Biomarkers in Hepatocellular Carcinoma: Diagnosis, Prognosis and Treatment Response Assessment. Cells. 2020;9(6).
Piscaglia F, Wilson SR, Lyshchik A, et al. American College of Radiology Contrast Enhanced Ultrasound Liver Imaging Reporting and Data System (CEUS LI-RADS) for the diagnosis of Hepatocellular Carcinoma: a pictorial essay. Ultraschall Med. 2017;38(3):320-324.
Qian K, Zhang F, Allison SK, Zheng C, Yang X. Image-guided locoregional non-intravascular interventional treatments for hepatocellular carcinoma: Current status. J Interv Med. 2021;4(1):1-7.
Qin S, Chen M, Cheng AL, et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): a randomised, open-label, multicentre, phase 3 trial. Lancet. 2023.
Reig M, Forner A, Rimola J, et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76(3):681-693.
Reveron-Thornton RF, Teng MLP, Lee EY, et al. Global and regional long-term survival following resection for HCC in the recent decade: A meta-analysis of 110 studies. Hepatol Commun. 2022;6(7):1813-1826.
Roayaie S, Schwartz JD, Sung MW, et al. Recurrence of hepatocellular carcinoma after liver transplant: patterns and prognosis. Liver Transpl. 2004;10(4):534-540.
Rubinstein MM, Kaubisch A, Kinkhabwala M, Reinus J, Liu Q, Chuy JW. Bridging therapy effectiveness in the treatment of hepatocellular carcinoma prior to orthotopic liver transplantation. J Gastrointest Oncol. 2017;8(6):1051-1055.
Sahu SK, Chawla YK, Dhiman RK, et al. Rupture of Hepatocellular Carcinoma: A Review of Literature. J Clin Exp Hepatol. 2019;9(2):245-256.
Salem R, Padia SA, Lam M, et al. Clinical, dosimetric, and reporting considerations for Y-90 glass microspheres in hepatocellular carcinoma: updated 2022 recommendations from an international multidisciplinary working group. Eur J Nucl Med Mol Imaging. 2023;50(2):328-343.
Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systematic Review and Meta-analysis. Ann Surg. 2021;273(4):656-666.
Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57(11):1592-1596.
Sun HC, Zhu XD. Downstaging Conversion Therapy in Patients With Initially Unresectable Advanced Hepatocellular Carcinoma: An Overview. Front Oncol. 2021;11:772195.
Uhlig J, Sellers CM, Stein SM, Kim HS. Radiofrequency ablation versus surgical resection of hepatocellular carcinoma: contemporary treatment trends and outcomes from the United States National Cancer Database. Eur Radiol. 2019;29(5):2679-2689.
Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2017;18(12):1624-1636.
Voesch S, Bitzer M, Blodt S, et al. Z Gastroenterol. 2022;60(1):e131-e185.
Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345-1362.
Wang L, Lin N, Lin K, et al. The Clinical Value of Postoperative Transarterial Chemoembolisation for Resectable Patients with Intermediate Hepatocellular Carcinoma After Radical Hepatectomy: a Propensity Score-Matching Study. J Gastrointest Surg. 2021;25(5):1172-1183.
Wang Y, Luo Q, Li Y, Deng S, Wei S, Li X. Radiofrequency ablation versus hepatic resection for small hepatocellular carcinomas: a meta-analysis of randomised and nonrandomised controlled trials. PloS one. 2014;9(1):e84484.
Weber M, Lam M, Chiesa C, et al. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2022;49(5):1682-1699.
Yan X, Huang S, Yang Y, et al. Sirolimus or Everolimus Improves Survival After Liver Transplantation for Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Transpl. 2022;28(6):1063-1077.
Yao FY, Ferrell L, Bass NM, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumour size limits does not adversely impact survival. Hepatology. 2001;33(6):1394-1403.
Yao FY, Mehta N, Flemming J, et al. Downstaging of hepatocellular cancer before liver transplant: long-term outcome compared to tumours within Milan criteria. Hepatology. 2015;61(6):1968-1977.
Yau T, Park JW, Finn RS, et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23(1):77-90.
Yau T, Tang VY, Yao TJ, Fan ST, Lo CM, Poon RT. Development of Hong Kong Liver Cancer staging system with treatment stratification for patients with hepatocellular carcinoma. Gastroenterology. 2014;146(7):1691-1700 e1693.
Zhou Y, Lei X, Wu L, Wu X, Xu D, Li B. Outcomes of hepatectomy for noncirrhotic hepatocellular carcinoma: a systematic review. Surg Oncol. 2014;23(4):236-242.
Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952.
Zhu AX, Kang YK, Yen CJ, et al. Ramucirumab after sorafenib in patients with advanced hepatocellular carcinoma and increased alpha-fetoprotein concentrations (REACH-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(2):282-296.
Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2015;16(7):859-870.
