13. Primary biliary cholangitis
Heike Bantel, Andreas E. Kremer
Abstract
Primary biliary cholangitis (PBC) is a chronic inflammatory cholestatic liver disease that can result in end-stage biliary cirrhosis and associated complications. Because of its characteristic autoantibody signature, immune-mediated biliary injury and genetic risk factors, PBC is considered as autoimmune liver disease, although immunosuppressive therapy is ineffective. Treatment strategies have therefore focused on cholestatic consequences and symptom burden. PBC-associated symptoms such as pruritus, sicca syndrome and fatigue as well as extrahepatic immune-mediated co-morbidities, e.g. thyreopathy or rheumatoid arthritis, can strongly impair quality of life. The diagnosis is based on serological detection of elevated cholestasis parameters, especially alkaline phosphatase, and PBC-specific anti-mitochondrial/anti-nuclear antibodies. Liver biopsy is only required if PBC is suspected despite negative autoantibody diagnostic or to clarify liver comorbidities, such as autoimmune hepatitis or metabolic dysfunction-associated steatotic liver disease. The treatment goal is to prevent disease progression with ursodeoxycholic acid (UDCA) as approved standard therapy of PBC. Treatment response should be assessed latest after 6 to 12 months of standard therapy by blood-based response criteria. In case of inadequate treatment response, a second line therapy with licensed novel PPAR agonists or off-label use of bezafibrate under continuation of UDCA therapy is possible for patients without decompensated cirrhosis. In summary, it is important to consider PBC in the differential diagnosis of cholestatic liver disease in order to enable early diagnosis of this rare liver disease and thus to create the best conditions for effective treatment and prevention of disease progression and complications.
Introduction
Primary biliary cholangitis is an immune-mediated, chronic cholestatic disease of the liver with a female predominance characterised by selective destruction of the small intrahepatic bile ducts resulting in non-suppurative destructing cholangitis (Lleo A 2020). It is characterised by anti-mitochondrial antibodies or PBC-specific anti-nuclear antibodies, progressive cholestasis, and characteristic histological features (European Association for the Study of the Liver 2017). Untreated, PBC can progress from lymphocytic cholangitis and progressive ductopenia to biliary cirrhosis (Lleo A 2020). In 2016, the term “primary biliary cirrhosis” was replaced by “primary biliary cholangitis” as currently most patients never develop cirrhosis anymore, as the cirrhosis stigmatised many patients, and caused association with alcohol consumption. The acronym PBC, however, remains unchanged (Beuers 2015).
The prevalence of PBC varies regionally and ranges between 1.9 and roughly 40 per 100,000 inhabitants (Liu 2010, Selmi 2011, Younossi 2019). The age at diagnosis is usually around the 5th decade of life, although cases have been described as early as the 2nd decade of life. In 90% of cases women are affected by the disease (Lu 2018). It is estimated that 0.5% of the normal population is AMA positive (Mattalia 1998). In a French prospective study, the 5-year PBC incidence rate for patients with positive AMA detection and normal AP was 16% (Dahlqvist 2017). The majority of these patients with a later diagnosis of PBC were women (89%) and the median age of diagnosis was 62 years. In another European long-term follow-up study in patients with AMA detection without further laboratory evidence of PBC at baseline, only about 10% developed PBC at follow-up > 6 years (Zandanell 2020). The probability of developing PBC was even lower in a retrospective Chinese study, with a 7.8% 5-year PBC incidence rate in patients with a positive AMA titer and no evidence of liver disease at the time of AMA detection (Duan W 2022).
Pathophysiology
The pathophysiology of primary biliary cholangitis is still not fully understood. It is believed that multiple endogenous and exogenous factors mutually interact and synergistically promote the initiation and progression of primary biliary cholangitis. The clinical observation of a broad array of immune-mediated phenomena suggests the disease to be of autoimmune aetiology. Still, the question remains whether pathophysiological alterations within and damage of the small intrahepatic biliary epithelial cells (BECs), such as dysfunction of the protective biliary bicarbonate-rich umbrella, cause a subsequent immune reaction or whether a direct immune attack, e.g. due to a molecular mimicry following an infection, results in the non-suppurative destructive cholangitis. A loss of immune tolerance to the E2 subunit of pyruvate dehydrogenase complex (PDC-E2), that is located on the inner membrane of mitochondria, results in a multi-lineage immune response. Despite the ubiquitous presence of mitochondria, selectively BECs within the small intrahepatic bile ducts are attacked by the immune system. Without therapy, this immune reaction results in a progressive and irreversible destruction and loss of small interlobular and septal bile ducts with formation of a biliary fibrosis and finally cirrhosis. Genome-wide association studies (GWAS) proofed important immunoregulatory pathways, such as IL-12 and IFN-γ (Hirschfield 2009, Gerussi 2021). More recently, a X-wide association study (XWAS) identified a risk locus on the X chromosome that underlines the importance of Treg cells (Asselta 2021). Finally, epigenetic modifications of the X chromosome could lead to closing the missing heritability gap and shed light on the biology of female predisposition.
Diagnosis of PBC
The diagnosis of PBC should be considered in the presence of elevated alkaline phosphatase (AP) after exclusion of obstructive (e.g. by ultrasound) and other causes of cholestatic liver diseases (Table 1), especially in women above the age of 30 who reveal a higher PBC prevalence compared to men (9:1 ratio) (Lu 2018). The diagnosis is confirmed if anti-mitochondrial antibodies (AMAs) can be detected in the presence of AP elevation. AMAs can be found in 90–95% of PBC patients (Vergani 2004). Approximately 5–10% of PBC patients remain AMA negative in immunofluorescence technique (Kaplan 2005). In some of those patients, antibodies against the major M2 components (pyruvate dehydrogenase complex-E2 and 2-oxoglutaric acid dehydrogenase complex) can be detected by ELISA or Western Blotting. More than 30% of PBC patients negative for AMA by indirect immunofluorescence technique reveal PBC-specific anti-nuclear antibodies (ANAs), including sp-100 and gp-210 (Vergani 2004; Hirschfield 2008). Therefore, in case of AMA-negativity, detection of PBC-specific ANAs by ELISA and immunofluorescence is recommended.
If PBC is suspected in patients negative for AMAs and PBC-specific ANAs, liver biopsy should be performed. PBC is histologically characterised by chronic, non-suppurative cholangitis and destruction of interlobular bile ducts. In addition, epithelioid granuloma can be observed. Inflammatory bile duct damage can result in ductopenia and collagen deposition (fibrosis development) which can be divided into four disease stages according to Ludwig and Scheuer (Ludwig 1978, Scheuer 1998). The four-stage histological model according to Nakanuma et al. considers cholestasis and bile duct loss in addition to fibrosis and might be of higher prognostic relevance compared to the classical PBC-staging (Nakanuma 2010).
The diagnosis PBC is confirmed when two of the three criteria – AP elevation, presence of AMAs or PBC-specific ANAs and histological characteristics of PBC – are met (Lindor 2019).
PBC-associated symptoms
Although PBC can be asymptomatic, a substantial proportion of patients develop symptoms, usually between 2 and 4 years following diagnosis (Roll 1983). The classical symptoms of PBC are fatigue, pruritus, and sicca syndrome, which affect more than 50% of patients (Hegade 2019, Mang 1997, Cauch-Dudek K 1998).
Fatigue can strongly impair quality of life and is not related to severity of liver disease with the exception of end-stage liver disease and associated hepatic encephalopathy (Poupon 2004; Huet 2000). Further causes of fatigue should be excluded, such as hypothyroidism, anaemia, coeliac disease, depression, nighttime pruritus, and sleep disturbance.
Pruritus represents a characteristic cholestatic symptom in PBC, which can occur at any disease stage and can dramatically impair quality of life (Beuers 2014). Patients with a ductopenic course of the disease being at risk for advanced disease progression are especially affected by severe pruritus (Vleggaar 2001). With respect to the increased risk of gall stones and associated complications in PBC, bile duct obstruction must be excluded (e.g. by ultrasound) as cause of pruritus.
Sicca symptoms, including dry eyes and / or mouth are typical signs in PBC and can reduce quality of life (Fox 2008). Most PBC patients have sicca-like symptoms without fulfillment of the characteristics of a primary Sjögren’s Syndrome. Enlargement of the parotid gland is, however, indicative of primary Sjögren’s Syndrome and should be further clarified.
As in other cholestatic liver diseases, hyperlipidaemia can also be observed in PBC and can be associated with xanthomas and xanthelasma. Increased levels of LDL cholesterol (mainly composed of non-artherogenic lipoprotein X) are usually not related with an increased cardiovascular risk in PBC patients if other further risk factors, such as arterial hypertension, diabetes mellitus, smoking or familial risk are excluded (Jahn 1985). In PBC patients with concomitant cardiovascular risk factors, however, lipid diagnostic and risk-adapted treatment of hyperlipidaemia are recommended (Cash 2013).
PBC patients have an increased risk of osteopenia and osteoporosis compared to age- and sex-matched controls, which can further negatively impact quality of life due to bone and joint alterations and associated pain (Menon 2001). Bone mineral density testing is therefore recommended at diagnosis and in regular follow-up intervals according to the individual risk (Lindor 2019). Musculoskeletal changes and associated discomfort can also be caused by rheumatologic comorbidities in PBC, which have to be considered in the diagnostic approach (Table 2).
| 1) Cholestatic Liver Diseases |
| Primary biliary cholangitis |
| Primary / secondary sclerosing cholangitis |
| IgG4-related cholangiopathy |
| Drug-/toxin-induced cholestasis |
| Viral cholangitis (e.g. CMV, HIV) |
| Obstructive cholangiopathy (e.g. bile duct stones, intra-/extra-hepatic malignancies) |
| Ductal plate malformation (e.g. Caroli syndrome) |
| Vanishing-bile-duct syndrome |
| Ischemic type biliary lesion (e.g. after liver transplantation) |
| Cystic fibrosis-associated cholangiopathy |
| Intrahepatic cholestasis of pregnancy |
| Hereditary cholestasis syndromes (e.g. FIC1-, BSEP- or MDR3-deficiency), Alagille syndrome, erythropoetic protoporphyria |
| Sarcoidosis, storage diseases, amyloidosis |
| Nodular regenerative hyperplasia |
Extrahepatic immune-mediated diseases in PBC
PBC patients reveal an increased risk for the development of extrahepatic immune-mediated diseases (Chalifoux 2017, Efe 2021, Floreani 2018, Gershwin 2005), such as Sjögren’s syndrome, Raynaud syndrome or systemic sclerosis/CREST (calcinosis, Raynaud’s phenomenon, oesophageal dysmotility, sclerodactyly and teleangectasia) syndrome, as well as rheumatoid arthritis and autoimmune thyroid diseases, especially Hashimoto thyroiditis (Table 2).
Table 2.| 2) Immune-mediated co-morbidities associated with PBC |
| Sjögren’s Syndrome |
| Autoimmune Thyreoiditis |
| Rheumatoid Arthritis |
| Systemic sclerosis / CREST syndrome |
| Raynaud Syndrome |
Risk factors of PBC progression and prognosis
Several risk factors are associated with disease progression and a higher risk of mortality or need for liver transplantation in PBC. For instance, younger age (< 50 years) at diagnosis of PBC is related with an unfavourable prognosis (Carbone 2013). Whereas AMA levels are not of prognostic relevance, detection of PBC-specific ANAs such as gp210 antibodies are associated with faster disease progression (Nakamura 2007), in particular in patients with an inadequate response to UDCA (Haldar 2021).
The efficacy of the standard therapy (UDCA) on disease progression has been proven by several placebo-controlled and long-term observational studies (Leuschner 1989, Poupon 1994, Poupon 1997, Corpechot 2000). A meta-analysis of the global PBC study group including 4845 PBC patients demonstrated that UDCA-treated patients reveal a significant better transplantation-free survival compared to untreated PBC patients (Lammers 2014). Patients with insufficient response to UDCA therapy, who does not fulfill biochemical response criteria (e.g. Paris-II criteria: AP and AST < 1.5-fold upper limit of normal and normal bilirubin after 12 month of UDCA treatment) have a worse prognosis compared to patients with treatment response (Corpechot 2011). Furthermore, transplantation-free survival of patients with early PBC responding to UDCA therapy is comparable with a healthy control population (Corpechot 2011, Pares 2006).
In PBC patients who received a liver biopsy, fibrosis stage and ductopenia (> 50% bile duct loss) are of prognostic relevance. Importantly, PBC patients with histologically progressed liver fibrosis (F3/F4) reveal a reduced transplantation-free survival independent of treatment response (Kumagi 2010, Murillo Perez 2019).
Prognostic scores from the UK PBC Research Group or the Global PBC Study Group (GLOBE score) can be used to estimate the individual risk of disease progression and the development of associated complications (Lammers 2015, Carbone 2016). These scores consider the disease stage (alkaline phosphatase, albumin, bilirubin and platelet count) as well as age at diagnosis as important risk factors (Gatselis 2020, Carbone 2013). A GLOBE score > 0.30 after one year of standard therapy is associated with a significant shorter transplantation-free survival of PBC patients compared to healthy individuals (Lammers 2015). In multicentre PBC cohorts, it could also be demonstrated that patients who achieved normalisation of AP or low-normal bilirubin levels (≤ 0.6-fold of upper limit of normal range) on standard therapy had significantly higher survival rates compared to patients with AP or bilirubin levels above this threshold (Murillo Perez 2020). Thus, at least in younger patients or those with risk factors normalisation of laboratory parameters is the novel, optimal treatment goal.
Risk stratification of PBC patients by liver stiffness measurement
In addition to blood-based parameters and scores, liver stiffness measurement is useful for risk stratification of PBC patients. Initially, a cut-off value of > 9.6 kPa could be identified for liver stiffness measurement by vibration-controlled transient elastography (VCTE) in PBC patients, which was associated with an increased risk for liver decompensation, mortality or need for liver transplantation (Corpechot 2012). A multicentre PBC study identified a similar VCTE cut-off value (10.2 kPa) for the prediction of liver decompensation independently from treatment response and GLOBE score (Osman 2021). A recent guideline from the European Association for the Study of the Liver (EASL) therefore recommends VCTE for risk stratification in PBC and suggests a threshold of 10 kPa for prediction of advanced fibrosis in PBC (EASL 2021). A further multicentre study identified a low (8 kPa) and a high (15 kPa) VCTE threshold for risk stratification of PBC patients in terms of low or high risk of developing clinical outcomes such as liver-related complications, liver transplantation or death (Corpechot 2022). Of note, body mass index and liver biochemistry did not affect liver stiffness measurement in PBC (Cristoferi 2021). Liver stiffness measurement therefore represents a reliable method for monitoring of PBC progression and further improved risk stratification in addition to GLOBE score or criteria of treatment response (Paris-II) (Corpechot 2012, Corpechot 2022).
Hepatocellular carcinoma risk and surveillance in PBC
PBC patients with liver cirrhosis have an increased risk for developing hepatocellular carcinoma (HCC), although the general risk is lower compared to end-stage liver diseases of other etiologies such as chronic hepatitis B or C virus infection (Natarajan 2021, Lindor 2019). In addition to progressed fibrosis, male sex, and inadequate treatment response have been identified as risk factors for HCC development in PBC. Surveillance with regular (bi-annual) imaging modalities and alpha fetoprotein (AFP) is recommended in international guidelines for patients with liver cirrhosis independently of aetiology and was associated with better clinical outcome of PBC patients who developed HCC (Lindor 2019, Bitzer 2023, Trivedi 2014, Silveira 2008).
Therapeutic options in PBC
First-line therapy
The drug of choice for slowing the progression of the disease is consistent drug therapy of PBC with the bile acid ursodeoxycholic acid (UDCA). Thus, UDCA is the recommended first-line therapy in all patients diagnosed with PBC. All patients should receive a dosage of 13-15 mg/kg body weight/day that should be titrated and divided into two daily doses at least during the first three months of therapy. According to current knowledge, lifelong therapy is indicated. UDCA is a physiological bile acid that accounts for up to 3% of the bile acid pool in healthy individuals. Oral supplementation will increase this proportion to around 50% (Beuers 2006). As an endogenous substance, UDCA is very well tolerated. Occasionally an increased stool frequency can occur, especially at the start of therapy, which is why a gradual dosage is recommended. Other side effects are rare and are generally due to additives in the drug. A change of preparation can therefore be tried in the event of intolerance. The mere detection of AMA, sp100 or gp210 in the absence of increased cholestasis parameters does not justify therapy.
Several placebo-controlled studies underline the efficacy of UDCA in patients with PBC (Poupon 1991, Poupon 1994, Poupon 1997, Lindor 1996, Lammert 2014). Prognostically important laboratory parameters such as AP, AST and bilirubin improved in these studies. Liver histology was also positively influenced and histological progression was delayed (Angulo 1999), while a smaller placebo-controlled study was unable to demonstrate any histological effects of UDCA treatment in PBC patients after 2 years (Batts 1996). In patients with advanced PBC, transplant-free survival improved (Angulo 1999). Patients with early-stage disease and a good response to UDCA did not differ in transplant-free survival from a healthy control population (Pares 2006, Corpechot 2011). The number needed to treat (NNT) without or with cirrhosis to avoid liver transplantation or death with UDCA is only 20 and 4, respectively. In patients with an AP above 4 times the norm, the NNT is 5 (Harms 2020). Long-term studies have demonstrated that this first-line treatment standard leads to a significant improvement in the prognosis of patients with PBC (EASL 2017). The proportion of patients requiring a PBC-related liver transplant fell from 20% in 1986 to 4% in 2015 compared to other indications for a liver transplant. The largest proportion of the decline took place from 1986 to 1996, after which the number of PBC patients receiving liver transplants remained largely stable (Harms 2019). This indicates a relevant group of patients in whom it is not possible to prevent advanced liver disease or in whom PBC was only diagnosed in the context of decompensation of liver cirrhosis. PBC patients requiring transplantation tend to be marginally older (56 vs. 54 years) than 30 years ago (Harms 2019).
The treatment response to UDCA is usually assessed after 6 or 12 months at the latest. Biochemical parameters are used as surrogate markers to define the treatment response, in particular alkaline phosphatase (AP), total bilirubin and transaminases (EASL 2017). They are part of several dichotomous criteria for UDCA response (e.g. Barcelona, Paris I or Paris II criteria) (Pares 2006, Corpechot 2008, Corpechot 2011) or continuous scoring systems (e.g. GLOBE score) (Lammers 2015), which were developed to define an inadequate UDCA response. Depending on the response criteria used, up to 30–40% of UDCA-treated patients have an inadequate response to treatment. These patients have an increased risk of developing liver cirrhosis (Carey 2015, EASL 2017). However, which response criteria best define an inadequate UDCA response and should be applied in everyday clinical practice is left open in the guidelines (Jones 2022). This heterogeneity can lead to uncertainties that make it difficult to identify patients at risk of disease progression. Correct risk stratification can also be difficult if the surrogate markers are in the borderline range between "low" and "high" risk of progression (Jones 2022). More recent data indicate that a response to first-line UDCA therapy can be defined even more narrowly: patients who achieve a low-normal bilirubin value of ≤ 0.6x ULN (upper limit of normal), or a normal-value AP (≤ 1x ULN) under UDCA therapy, have the lowest risk of liver transplantation or death after 10 years (Murillo Perez 2020). A rule of thumb can be derived from this, which should be easier to apply for risk stratification in everyday clinical practice (Figure 1): According to this, normalisation of AP and bilirubin should be aimed for under UDCA (Murillo Perez 2020). If this aim is not achieved with UDCA, there are below standing second-line treatment options available (Figure 1).
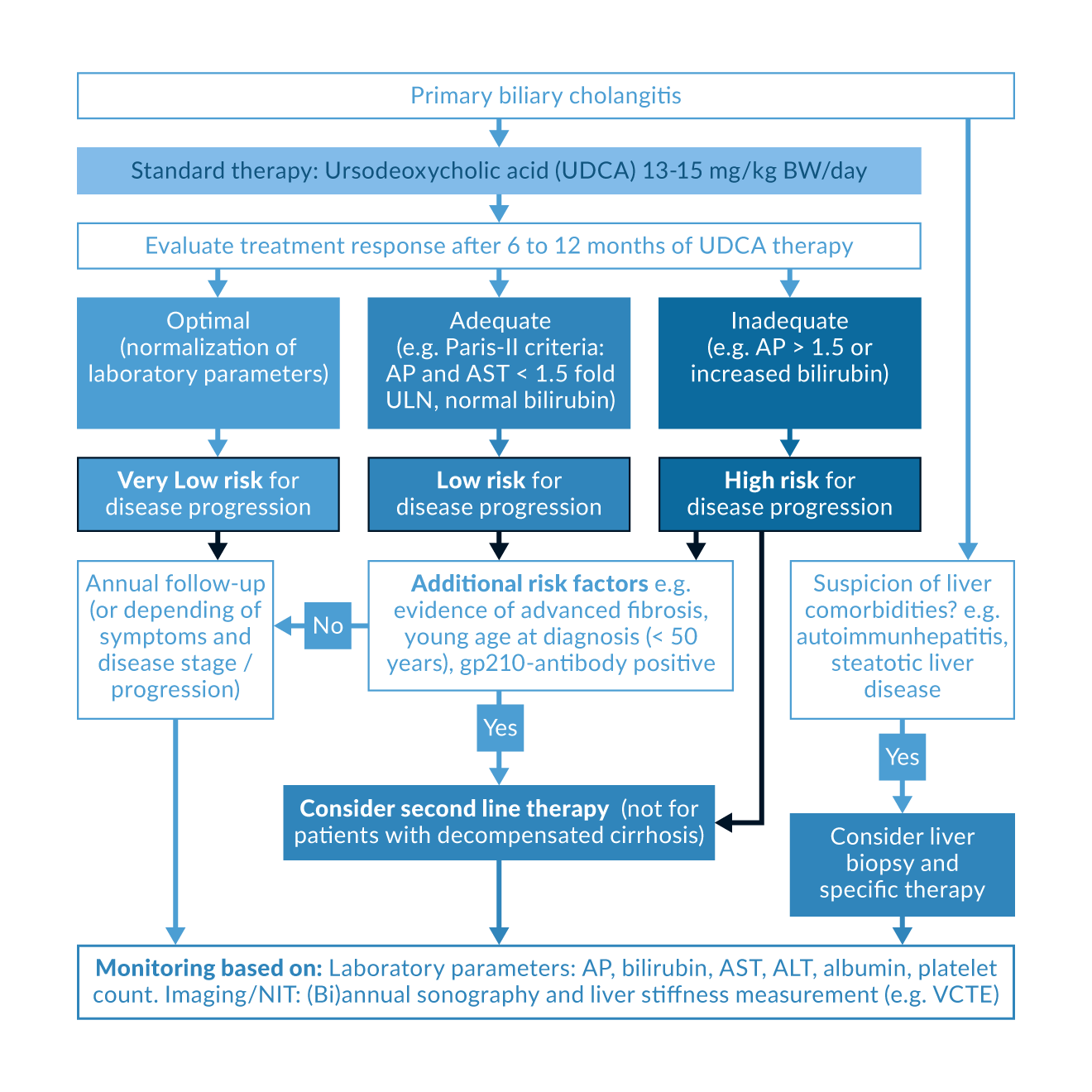 Figure 1. Management of PBC patients according to standard therapy response. NIT, non-invasive test; VCTE, vibration-controlled transient elastography; AP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Figure 1. Management of PBC patients according to standard therapy response. NIT, non-invasive test; VCTE, vibration-controlled transient elastography; AP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ULN, upper limit of normal.
Second-line therapy
In 2016, obeticholic acid (OCA) was approved as second-line therapy in combination with UDCA for patients with PBC who do not respond adequately to UDCA, or – clinically necessary in only a few cases – as monotherapy in cases of UDCA intolerance (Nevens 2016). OCA is a semisynthetic bile acid that represents the first highly selective and high-affinity first-in-class agonist of the nuclear farnesoid X receptor (FXR). It exhibits anti-cholestatic, anti-inflammatory, anti-fibrotic and hepatoprotective properties (Forman 1995, Gadaleta 2011). The clinical data on OCA is very extensive and includes 2 dose-finding studies (Hirschfield 2015; Kowdley 2018), a phase 3 study (POISE) (Nevens 2016) plus data from an open extension phase (POISE LTSE) (Trauner 2019). The primary combined endpoint of the POISE study was defined as biochemical response with an AP < 1.67x ULN, an AP reduction of at least 15% and normalisation of total bilirubin (Nevens 2016). The proportion of patients who achieved the primary combined endpoint after 12 months with OCA was 46% (5-10 mg/day) and 47% (10 mg/day), respectively, compared to 10% in the placebo arm (Nevens 2016). The treatment response was maintained even after 72 months of long-term treatment with OCA (Nevens 2019). The proportion of patients reaching the primary endpoint increased to over 50 % and led to significant, sustained reductions in AP, GGT and transaminases (ALT, AST). Bilirubin levels remained stable over 6 years. Liver stiffness stabilised in all subgroups of patients in the same period and there were no new safety signals with OCA therapy. Treatment discontinuation due to pruritus occurred in only 4% of patients during the extension phase (Nevens 2019, Trauner 2019). Finally, in a subgroup of patients (n=16) in whom paired liver biopsies were available (before the start of the double-blind study and after approximately 3 years of OCA therapy) collagen morphometry was clearly indicated to be improved in a post-hoc analysis (Bowlus 2020).
There are also real-world studies in international patient cohorts underlining the efficacy and safety of OCA (Roberts 2020, D`Amato 2021, Abbas N 2023). In addition, a recently published comparative analysis also provided important evidence for the improvement of clinical endpoints under OCA: using the propensity matching method, OCA-treated patients from the POISE study (6-year follow-up) were compared with UDCA-treated patients from two real-world cohorts. In the cross-study comparison, treatment with OCA resulted in significantly superior transplant-free survival (HR [hazard ratio]: 0.29 [95% CI: 0.10–0.83] for POISE vs. global PBC and HR: 0.30 [95% CI: 0.12–0.75] for POISE vs. UK PBC) (Murillo Perez 2022).
The FDA has recommended a contraindication in decompensated liver cirrhosis and also in compensated liver cirrhosis with signs of portal hypertension. These recommendations are based on case reports of acute liver failure, presumably as a result of an OCA overdose (Eaton 2020). In June 2024, EMA recommended revoking conditional marketing authorisation of OCA as the benefits would no longer outweigh its risks. This decision is based on the results of the COBALT study (Phase 3b; OCA vs. placebo over 8 years), which did not show significant differences in the primary composite endpoint, i.e. time to death, liver transplant, MELD score ≥ 15 or hepatic decompensation, between the treatment groups. However, it should be mentioned that many drop-outs and therapeutic switches of patients in the placebo group due to insufficient treatment response and ethical reasons confounded the results (Jones 2024, Kowdley 2022). In November 2024, OCA finally lost its conditional marketing authorisation in the European Union.
Fibrates are nuclear peroxisome proliferator activated receptor (PPAR) agonists that are approved for the treatment of hyperlipidaemia. A meta-analysis of phase 2 studies and several cohort studies indicated that a combination therapy of fenofibrate or bezafibrate and UDCA significantly reduced biochemical markers such as AP and serum bilirubin in patients with an inadequate response to UDCA (Honda 2013, Honda 2019, Grigorian 2015, Tanaka 2015). In the BEZURSO trial, a randomised phase 3 study, 100 patients who had not adequately responded to UDCA therapy according to the Paris II criteria were treated either with a combination of 400 mg bezafibrate per day and UDCA or with UDCA and placebo. The endpoint of normal AST, ALT, AP, modified prothrombin time and normal bilirubin was achieved in 31% of patients in the bezafibrate group compared to 0% in the placebo group. Two thirds of the bezafibrate-treated patients exhibited a complete normalisation of their AP values. Furthermore, a decrease in liver stiffness and some benefit in pruritus were observed (Corpechot C 2018). In a Japanese cohort, transplant-free survival also improved with bezafibrate (Tanaka A 2021). A smaller, non-randomised study reported that additional fibrate therapy in PBC patients with an inadequate response to UDCA can reduce the risk of developing liver cirrhosis or liver decompensation (Chung 2019). In addition to the low costs, the anti-pruritogenic effect of bezafibrate speaks for its use. However, fibrates are not approved for PBC therapy.
Side effects of fibrates include myalgia, gastrointestinal complaints, and increase in retention parameters; hepatotoxicity was observed in 6–10% of cases, some of which required steroid therapy (Corpechot 2018, Abbas N 2023). Appropriate monitoring under bezafibrate is therefore necessary. Furthermore, bezafibrate should be applied with caution in patients with advanced disease stages and is not recommended in cases of portal hypertension, decompensated liver cirrhosis, or impaired renal function.
There are few but promising data on the possible additive effect of triple therapy with UDCA, OCA and fibrates in PBC patients who have not responded adequately to second-line therapy (UDCA+OCA or UDCA+fibrate) (Smets 2021, Soret 2021, Reig 2021). First data from two randomised, placebo-controlled, phase 2, dose-finding studies have further indicated the stronger anti-cholestatic benefit for directly combining obeticholic acid plus bezafibrate (Hejda 2023, Levy 2023a). Larger phase 3 trials and clinical end point studies are warranted to support such an approach in the real-life setting. However, it remains unclear whether OCA will receive a new marketing authorisation for this application.
Currently, there are three further PPAR agonists, elafibranor, seladelpar, and saroglitazar being investigated as second line therapy in combination with UDCA in large, randomised, placebo-controlled phase 3 studies. The PPARα/δ agonist elafibranor at a daily dose of 80 mg achieved the primary endpoint (defined as an AP < 1.67x ULN, with a reduction of ≥ 15% from baseline, and normal total bilirubin levels at week 52) in 51% of patients compared to 4% in the placebo arm. Fifteen percent of elafibranor-treated patients achieved AP normalisation (Kowdley 2024). In October 2024, elafibranor received conditional marketing authorisation in the European Union for the treatment of PBC patients insufficiently responding to UDCA therapy. The selective PPARδ agonist seladelpar achieved at a daily dose of 10 mg the same primary endpoint (month 12) in 61.7% compared to 20% in the placebo arm. Seladelpar (10 mg/day) resulted in AP normalisation in 25% of patients compared to 0% in the placebo group (Hirschfield 2024). Furthermore, the benefit of pruritus improvement seems stronger under seladelpar therapy compared to elafibranor. European Union marketing authorisation of seladelpar for second line treatment of PBC was granted February 2025. Data on the phase 3 study of the PPARα/γ agonist saroglitazar have not been published to date. These new therapeutic options will significantly diversify the treatment options for PBC patients in the future. In addition to the laboratory response, effective symptom control will move into the focus of future therapeutic developments.
Extrahepatic manifestations and quality of life
Around 60% of patients are discovered as a result of abnormal laboratory findings and are often still asymptomatic at the time of diagnosis. According to a larger cohort analysis (n = 770) from England, however, the prognosis of PBC is not better if the patients are symptom-free at the time of diagnosis. In addition, patients almost invariably become symptomatic with increasing disease duration, with around 95% becoming symptomatic after 20 years (Prince 2004). In PBC, the severity of the clinical symptoms does not correlate with the stage of the underlying disease. Treatment with UDCA generally does not improve the symptoms of pruritus, fatigue and sicca syndrome (EASL 2017; Hirschfield 2018).
Patients with PBC often suffer from a wide range of different symptoms. Extrahepatic manifestations can lead to a significant reduction in quality of life (Mells 2013). The most common primary extrahepatic symptoms of PBC include fatigue and pruritus. The world's largest PBC patient cohort on symptom burden to date is the UK-PBC National Study Cohort with 2353 patients. Fatigue and symptoms of social dysfunction were independently associated with reduced quality of life (Mells 2013). The younger the patients were, the more their quality of life was impaired (Dyson 2016). In a study on the perception of quality of life, female patients with PBC rated their quality of life even lower than female patients with type II diabetes mellitus (Untas 2015). Even stronger, PBC patients with severe pruritus had a comparable EQ-5D quality of life measure compared to severe Parkinson`s Disease (Smith 2025). Against this background, the European guideline on the diagnosis and treatment management of patients with PBC emphasises the importance of clinical symptoms. It recommends an "active evaluation" and "active management of PBC-associated symptoms", namely pruritus, fatigue, sicca symptoms, bone changes and comorbid autoimmune diseases (EASL 2017).
Pruritus
For many patients, pruritus is particularly agonising and significantly impairs their quality of life (EASL 2017; Düll 2019). Pruritus is considered chronic if it persists for longer than 6 weeks. In the largest collection of data to date on cholestatic pruritus in 2194 PBC patients, 73% of patients reported pruritus at some point during the course of the disease and 34% reported chronic persistent pruritus. At the onset of PBC, pruritus was already moderate to severe in 28% of patients. However, almost half of the patients with severe pruritus had not yet received a single guideline-oriented drug therapy (Hegade 2019).
With increasing duration, pruritus persists independently of the underlying disease and acquires independent disease value as chronic pruritus. Cholestatic pruritus is often characterised by a circadian rhythm and increases significantly at night and in warm weather. Women often experience an increase in symptoms depending on their menstrual cycle, during late pregnancy or under hormone replacement therapy. The extremities are most frequently affected, especially the palms of the hands and soles of the feet (Beuers 2014). Severe courses restrict everyday activities, lead to a lack of sleep and thus to an increase in existing fatigue. This leads to adjustment, anxiety and depressive disorders, and even suicide (Tajiri 2017; Ständer 2022). In practice, regular recording of the symptom using a numerical rating scale (NRS) or verbal rating scale (VRS) is recommended for the assessment of the course (Ständer 2013). The psychological burden of pruritus should not be underestimated. Patients should therefore be specifically asked about their quality of life and sleep.
Current guidelines recommend a structured approach to pruritus management in stages. As a first step, patients are advised on basic therapeutic measures with moisturising and hydrating skin care products. Potential ingredients include urea, menthol, camphor, lidocaine, polidocanol or calcineurin inhibitors (off-label use). Topical therapy can be used alone or in combination with systemic therapies and/or UV phototherapy. Antihistamines are not recommended as a specific therapy, even though they can occasionally have non-specific antipruritic effects (EASL 2017). With the exception of the anion exchange resin cholestyramine, none of the systemic treatment options mentioned below are approved for cholestatic pruritus.
The side effect profile is considered favourable with adequate patient education, but the antipruritic efficacy of cholestyramine has only been investigated in small, uncontrolled studies (EASL 2017, Düll 2022). The recommended dose ranges from 4–16 g/d, 2-4 hours before or after other medications (EASL 2017). Patients should be carefully informed about possible interactions of the exchange resin with other drugs taken at the same time such as UDCA, digitoxin, oral anticoagulants and fat-soluble vitamins. As an alternative first-line off-label therapy, bezafibrate is recommended at a dose of 400 mg/day. The antipruritic efficacy of bezafibrate was underlined in the randomised, placebo-controlled study FITCH, in which 45% of the study participants achieved at least a 50% reduction in pruritus intensity within 3 weeks (de Vries 2021). Further case series also reported beneficial effects of bezafibrate on pruritus (Reig 2018) and in the BEZURSO-Trial bezafibrate had a trend towards improved pruritus albeit the baseline itch intensity was low with a NRS of 1 (Corpechot 2018). For potential adverse events see paragraph on second-line therapies.
If cholestyramine and/or bezafibrate is not tolerated or ineffective after two to four weeks, the anti-tuberculosis antibiotic and PXR-agonist rifampicin is considered as second-line therapy. The antipruritic efficacy of rifampicin was demonstrated in 4 prospective, randomised and placebo-controlled studies and confirmed by meta-analyses (EASL 2017, Tandon 2007). In most cases, low doses of 150–300 mg/day are sufficient for effective relief of pruritus. There are also increased interaction risks with rifampicin, e.g. with oral anticoagulants, oral contraceptives or antiepileptics. In a large real-life cohort of over 100 patients, treatment-induced hepatitis with liver dysfunction was observed in 5% of patients, indicating that transaminases should be monitored after 2, 6 and 12 weeks after start of therapy or dose modifications (Webb 2018). Patients should also be made aware of an orange-reddish discoloration of the urine and tear fluid.
Further anti-pruritic treatment options are the orally available opioid receptor antagonist naltrexone that was associated with moderate antipruritic effects in pruritus in randomised controlled trials and case reports (EASL 2017, Düll 2022). However, to avoid opioid withdrawal-like symptoms, naltrexone should be dosed gradually up to a dose of 50–100 mg/day. In particular for hospitalised patients or patients with decompensated liver cirrhosis, intravenous naloxone (0.002–0.2 μg/kg bw/min) represents a suitable alternative (Ständer 2022). An antipruritic effect has also being discussed for anticonvulsants such as gabapentin and pregabalin. They are preferably recommended for nephrogenic and neuropathic pruritus, but can also be considered for pruritus of other origins according to guideline recommendations (EASL 2017, Ständer 2022, Düll 2022). Selective serotonin reuptake inhibitors (SSRIs) such as sertraline (50-100 mg/day) are used empirically in patients in whom previous treatment attempts have failed: The available evidence on the efficacy of sertraline in cholestatic pruritus is limited to small individual studies (Browning 2003, Mayo 2007). The tetracyclic antidepressant mirtazapine (7.5–30 mg/day) with additional H1-antihistaminergic and serotonin-antagonistic effects is not listed in the European PBC guideline, but has been described as antipruritic in case series in cholestasis (Davis 2003). Mirtazapine can therefore be used as further off-label use option in the evening at doses from 7.5-30 mg/day (Ständer 2022).
Novel anti-pruritic therapeutic approaches that are currently investigated in phase 2 and 3 randomised placebo-controlled trials in PBC are ileal bile acid transporter (IBAT) inhibitors (Levy 2023b), Mas-related G protein-coupled receptor (MRGPR) X4 antagonists and κ-opioid receptor (KOR) agonists (Fishbane 2020, Düll 2022).
Invasive, experimental approaches that have been published in case reports also include extracorporeal albumin dialysis, plasmapheresis and biliary drainage using a nasobiliary tube. Case reports also describe positive effects of physical therapy measures such as UV phototherapy (UVA, UVB) or bright light therapy (EASL 2017, Ständer 2022, Düll 2022).
Fatigue
Fatigue also occurs regardless of the severity of the liver disease and can significantly impair the quality of life of patients with PBC. More than half of patients with PBC report fatigue, 20% of which is severe (EASL 2017). Fatigue should not be confused with chronic fatigue syndrome (CFS) that refers to a feeling of persistent exhaustion, the inability to cope with everyday activities and reduced mental and physical performance. Other internal causes such as hypothyroidism, anaemia, celiac disease, and heart failure or medication side effects such as antihistamines and beta blockers should be considered in the differential diagnosis. Severe, especially nocturnal pruritus with sleep disturbances can also contribute significantly to fatigue. Successful relief of nocturnal pruritus also improves the symptoms of fatigue. The exact pathomechanisms of this complex syndrome with peripheral and central components are not yet understood (EASL 2017).
There are still no specific or approved intervention options available. There is also no evidence that treating the underlying disease with UDCA improves fatigue. In particularly severe cases, treatment with the centrally acting sympathomimetic modafenil, which is approved for the treatment of narcolepsy, may be considered (EASL 2017). However, treatment with modafenil in patients with PBC-associated fatigue exhibited no demonstrable benefit over placebo in a randomised, double-blind study (Silveira 2017). Fatigue is not an indication for liver transplantation, as, unlike pruritus, fatigue is usually not significantly improved. Patients can benefit from a structured, multidisciplinary and integrated approach to improve quality of life (including fatigue) (Jones 2008) as well as learning coping strategies and avoiding social isolation (EASL 2017). Novel treatment approaches may include the blockade of NADPH oxidase 1/4 inhibitors as a post-hoc analysis of a phase 2 study using setanaxib in PBC indicated a benefit in patients with moderate to severe fatigue (Jones 2023).
Sicca symptoms
Patients with PBC often complain of sicca symptoms. The dryness can affect almost all mucous membranes, most frequently in the eye and/or mouth area. Keratoconjunctivitis sicca is treated symptomatically with tear substitutes and, in the case of refractory symptoms, additionally with eye drops containing parasympathomimetics such as pilocarpine or cevimeline. Pronounced dry mouth (xerostomia) leads to problems with prolonged speaking and chewing. Due to the increased risk of tooth decay, which is around twice as prevalent as in the general population, those affected should be encouraged to pay more attention to oral hygiene. In addition, the risk of oral candidiasis is 10fold higher in this patient group (EASL 2017). Mouth sprays containing carmellose can provide subjective relief, while chewing gum and lozenges stimulate saliva production. Vaginal moisturisers are available for women with vaginal sicca syndrome – however, local hormone-containing substances should only be prescribed in consultation with a gynecologist (EASL 2017).
Bone health: osteopenia and osteoporosis
The majority of patients with PBC have reduced bone density (Hirschfield 2018). In a Spanish study (185 women with PBC), the prevalence of osteoporosis (T-score: -2.5) in the lumbar spine was 30.6% compared to 11.2 % in an age-matched healthy control population. Overall, 37% of patients with PBC had developed osteoporosis (Guanabens 2010). Furthermore, an increased rate of bone fractures was observed with elafibranor (6%) and seladelpar (4%) compared to 0% in the placebo groups (see FDA prescribing information for elafibranor and seladelpar 2024). The European PBC guideline recommends considering the risk of osteoporosis in all PBC patients (EASL 2017). To this end, osteodensitometry should be performed at the time of diagnosis. Depending on the extent of the cholestasis and the individual risk profile, this should be repeated approximately every 1–5 years (EASL 2017). In patients with normal nutritional status and lack of features of calcium malabsorption calcium supplementation is not recommended (EASL 2017). Nevertheless, care should be taken to ensure sufficient calcium intake (1000 mg/day). In the absence of contraindications (e.g. history of kidney stones), primary prophylactic substitution with 25-OH vitamin D3 (1000 IU/day) or 20`000 IU/every second week can be given to increase the success of the intake and to achieve normal vitamin D levels in serum (Lindor 2019). For the treatment of osteoporosis, reference is made to corresponding guidelines. In case of intolerance to anti-resorptive bisphosphonate therapy, the involvement of osteoporosis specialists is recommended (Hirschfield 2018).
Cholestasis and nutritional advice
In the case of pronounced PBC-associated cholestasis, the risk of malabsorption of lipids and fat-soluble vitamins increases (EASL 2017). However, a manifest deficiency of fat-soluble vitamins is not commonly observed. Nevertheless, a significant number of patients with PBC exhibit reduced 25-OH vitamin D3 levels. Supplementation of fat-soluble vitamins should therefore be done on an individual basis (EASL 2017). Parenteral vitamin K supplementation may be considered in cases of impaired coagulation prior to surgery (EASL 2017).
The hypercholesterolaemia that regularly occurs in patients with PBC does not generally require treatment. The underlying mechanisms in PBC appear to differ from other cardiovascular risk diseases as lipoprotein X (LpX) is typically increased. The LpX fraction runs within the LDL fraction which results in false high LDL levels. However, LpX seems not to be of artherosclerotic potential (Longo 2002, Mach 2020). Only in case of additional cardiovascular risk such as arterial hypertension, diabetes mellitus or smoking, cholesterol-lowering pharmacological therapy should be applied (EASL 2017). The recommendations from the current European guidelines on dyslipidaemia management can be used for a risk-adapted approach in everyday practice (Mach F 2020).
Outlook
The treatment landscape for PBC is developing very positively. The pipeline of further second-line therapies is rapidly expanding, with further PPAR agonists and a first in class NOX1/4-inhibitor entering phase 3 trials. Thus, it will be possible to aspire for normal liver biochemistry, low symptom burden, and avoidance of liver transplantation. Beyond the classical anti-cholestatic and anti-inflammatory treatment regimen, there are currently also attempts at inducing immune tolerance to an encapsulated PDC-E2 antigen and reprograming the immune system (NCT05104853). Treatment of pruritus will further be strengthened by novel PPAR agonists but also by approaches targeting IBAT, KOR and MRGPRX4. The approval of drug for the treatment of symptom burden will further increase awareness of the symptom burden and the need for appropriate treatment of affected patients. Solely fatigue remains a difficult-to-treat symptom if moderate to severe. Novel approaches may include the NOX1/4-inhibitor setanaxib or golexanolone antagonising neurosteroids at the GABA receptor level. In summary, the future for patients with PBC is promising, remains dynamic and will improve the lives of many patients.
Key Messages
- Diagnosis of PBC should be considered in case of elevated alkaline phosphatase (AP) levels after imaging-based exclusion of obstructive cholestasis.
- PBC diagnosis is based on elevated AP levels in the presence of PBC-specific anti-mitochondrial or anti-nuclear antibodies or in case of non-detectable autoantibodies and PBC compatible liver histology.
- Ursodeoxycholic acid (UDCA 13–15 mg/kg bw) represents the standard first-line therapy in PBC and is of prognostic relevance.
- Treatment response is evaluated after 6 to 12 months of UDCA therapy by using response criteria based on laboratory parameters, e.g. AP, AST < 1.5x ULN and normal bilirubin (Paris-II response criteria).
- In case of inadequate response to the standard therapy, second line therapy with licensed novel PPAR agonists or with bezafibrate (off-label use) in addition to UDCA should be considered for PBC patients without decompensated liver cirrhosis.
- Monitoring of treatment efficacy and disease progression by laboratory parameters and, if available, by liver stiffness measurement is recommended.
- Monitoring of symptom burden such as fatigue, pruritus and sicca-syndrom should be performed regularly and treated consequently.
References
Abbas N, Culver EL, Thorburn D, et al. UK-wide multicenter evaluation of second-line therapies in primary biliary cholangitis. Clin Gastroenterol Hepatol. 2023;21(6):1561-1570.
Angulo P, Batts KP, Therneau TM, Jorgensen RA, Dickson ER, Lindor KD. Long-term ursodeoxycholic acid delays histological progression in primary biliary cirrhosis. Hepatology 1999;29(3):644-647.
Asselta R, Paraboschi EM, Gerussi A, et al. X chromosome contribution to the genetic architecture of primary biliary cholangitis. Gastroenterology 2021;160(7):2483-2495.
Batts KP, Jorgensen RA, Dickson ER, Lindor KD. Effects of ursodeoxycholic acid on hepatic inflammation and histological stage in patients with primary biliary cirrhosis. Am J Gastroenterol. 1996;91(11):2314-2317.
Beuers U. Drug insight: Mechanisms and sites of action of ursodeoxycholic acid in cholestasis. Nat Clin Pract Gastroenterol Hepatol. 2006;3(6):318-328.
Beuers U, Kremer AE, Bolier R, Elferink RP. Pruritus in cholestasis: facts and fiction. Hepatology 2014;60:399-407.
Beuers U, Gershwin ME, Gish RG, et al. Changing nomenclature for PBC: From 'cirrhosis' to 'cholangitis'. J Hepatol. 2015;63(5):1285-1287.
Bitzer M, Groß S, Albert J, et al. S3-Leitlinie Diagnostik und Therapie biliärer Karzinome. Z Gastroenterol. 2023;61(4):420-440.
Bowlus CL, Pockros PJ, Kremer AE, et al. Long-term obeticholic acid therapy improves histological endpoints in patients with primary biliary cholangitis. Clin Gastroenterol Hepatol. 2020;18(5):1170-1178.
Browning J, Combes B, Mayo MJ. Long-term efficacy of sertraline as a treatment for cholestatic pruritus in patients with primary biliary cirrhosis. Am J Gastroenterol. 2003;98(12):2736-2741.
Carbone M, Mells GF, Pells G, et al. Sex and age are determinants of the clinical phenotype of primary biliary cirrhosis and response to ursodeoxycholic acid. Gastroenterology 2013;144:560-569.
Carbone M, Sharp SJ, Flack S, et al. The UK-PBC risk scores: Derivation and validation of a scoring system for long-term prediction of end-stage liver disease in primary biliary cholangitis. Hepatology 2016;63(3):930-950.
Carey EJ, Ali AH, Lindor KD. Primary biliary cirrhosis. Lancet 2015;386(10003):1565-1575.
Cauch-Dudek K, Abbey S, Stewart DE et al. Fatigue in primary biliary cirrhosis. Gut 1998;43:705-710.
Cash WJ, O'Neill S, O'Donnell ME et al. Randomized controlled trial assessing the effect of simvastatin in primary biliary cirrhosis. Liver Int. 2013;33:1166-1174.
Chalifoux SL, Konyn PG, Choi G et al. Extrahepatic manifestations of primary biliary cholangitis. Gut Liver 2017;11:771-780.
Cauch-Dudek K, Abbey S, Stewart DE, Heathcote EJ. Fatigue in primary biliary cirrhosis. Gut 1998;43(5):705-710.
Chung SW, Lee JH, Kim MA, et al. Additional fibrate treatment in UDCA-refractory PBC patients. Liver Int. 2019;39(9):1776-1785.
Corpechot C, Carrat F, Bonnand AM, Poupon RE, Poupon R. The effect of ursodeoxycholic acid therapy on liver fibrosis progression in primary biliary cirrhosis. Hepatology 2000;32(6):1196-1199.
Corpechot C, Abenavoli L, Rabahi N, et al. Biochemical response to ursodeoxycholic acid and long-term prognosis in primary biliary cirrhosis. Hepatology 2008;48(3):871-877.
Corpechot C, Chazouilleres O, Poupon R. Early primary biliary cirrhosis: biochemical response to treatment and prediction of long-term outcome. J Hepatol. 2011;55:1361-1367.
Corpechot C, Carrat F, Poujol-Robert A, et al. Noninvasive elastography-based assessment of liver fibrosis progression and prognosis in primary biliary cirrhosis. Hepatology 2012;56(1):198-208.
Corpechot C, Chazouillères O, Rousseau A, et al. A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N Engl J Med 2018;378(23):2171-2181.
Corpechot C, Carrat F, Gaouar F, et al. Liver stiffness measurement by vibration-controlled transient elastography improves outcome prediction in primary biliary cholangitis. J Hepatol. 2022;77:1545-1553.
Cristoferi L, Calvaruso V, Overi D, et al. Accuracy of transient elastography in assessing fibrosis at diagnosis in naïve patients with primary biliary cholangitis: a dual cut-off approach. Hepatology 2021;74(3):1496-1508.
Dahlqvist G, Gaouar F, Carrat F, et al. Large-scale characterization study of patients with antimitochondrial antibodies but nonestablished primary biliary cholangitis. Hepatology 2017;65(1):152-163.
D'Amato D, De Vincentis A, Malinverno F, et al. Real-world experience with obeticholic acid in patients with primary biliary cholangitis. JHEP Rep. 2021;3(2):100248.
Davis MP, Frandsen JL, Walsh D, Andresen S, Taylor S. Mirtazapine for pruritus. J Pain Symptom Manage. 2003;25(3):288-291.
de Vries E, Bolier R, Goet J, et al. Fibrates for itch (FITCH) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial. Gastroenterology 2021;160(3):734-743.
Duan W, Chen S, Li S, et al. The future risk of primary biliary cholangitis (PBC) is low among patients with incidental anti-mitochondrial antibodies but without baseline PBC. Hepatol Commun. 2022;6(11):3112-3119.
Düll MM, Kremer AE. Treatment of pruritus secondary to liver disease. Curr Gastroenterol Rep. 2019;21(9):48.
Düll MM, Kremer AE. Evaluation and management of pruritus in primary biliary cholangitis. Clin Liver Dis. 2022;26(4):727-745.
Dyson JK, Wilkinson N, Jopson L, et al. The inter-relationship of symptom severity and quality of life in 2055 patients with primary biliary cholangitis. Aliment Pharmacol Ther. 2016;44(10):1039-1050.
Eaton JE, Vuppalanchi R, Reddy R, Sathapathy S, Ali B, Kamath PS.
Liver injury in patients with cholestatic liver disease treated with obeticholic ccid. Hepatology 2020;71(4):1511-1514.
Efe C, Torgutalp M, Henriksson I et al. Extrahepatic autoimmune diseases in primary biliary cholangitis: Prevalence and significance for clinical presentation and disease outcome. J Gastroenterol Hepatol. 2021;36:936-942.
European Association for the Study of the Liver. EASL clinical practice guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75:659-689.
European Association for the Study of the Liver. EASL clinical practice guidelines: the diagnosis and management of patients with primary biliary cholangitis. J Hepatol. 2017;67:145-172.
Fishbane S, Mathur V, Germain MJ, et al. Randomized controlled trial of difelikefalin for chronic pruritus in hemodialysis patients. Kidney Int Rep. 2020;5(5):600-610.
Floreani A, Cazzagon N. PBC and related extrahepatic diseases. Best Pract Res Clin Gastroenterol. 2018;34-35:49-54.
Forman BM, Goode E, Chen J, et al. Identification of a nuclear receptor that is activated by farnesol metabolites. Cell 1995;81(5):687-93.
Fox PC, Bowman SJ, Segal B et al. Oral involvement in primary sjogren syndrome. J Am Dent Assoc. 2008;139:1592-1601.
Gadaleta RM, van Erpecum KJ, Oldenburg B, et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011;60(4):463-472.
Gatselis NK, Goet JC, Zachou K et al. Factors associated with progression and outcomes of early stage primary biliary cholangitis. Clin Gastroenterol Hepatol. 2020;18:684-692 e686.
Gershwin ME, Selmi C, Worman HJ et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology 2005;42:1194-1202.
Gerussi A, Carbone M, Corpechot C, Schramm C, Asselta R, Invernizzi P. The genetic architecture of primary biliary cholangitis. Eur J Med Genet. 2021;64(9):104292.
Grigorian AY, Mardini HE, Corpechot C, Poupon R, Levy C. Fenofibrate is effective adjunctive therapy in the treatment of primary biliary cirrhosis: A meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39(3):296-306.
Guañabens N, Cerdá D, Monegal A, et al. Low bone mass and severity of cholestasis affect fracture risk in patients with primary biliary cirrhosis. Gastroenterology 2010 ;138(7):2348-2356.
Haldar D, Janmohamed A, Plant T, et al. Antibodies to gp210 and understanding risk in patients with primary biliary cholangitis. Liver Int 2021;41(3):535-544.
Harms MH, Janssen QP, Adam R, et al. Trends in liver transplantation for primary biliary cholangitis in Europe over the past three decades. Aliment Pharmacol Ther. 2019;49(3):285-295.
Harms MH, de Veer RC, Lammers WJ, et al. Number needed to treat with ursodeoxycholic acid therapy to prevent liver transplantation or death in primary biliary cholangitis. Gut 2020;69(8):1502-1509.
Hegade VS, Mells GF, Fisher H et al. Pruritus Is Common and Undertreated in Patients With Primary Biliary Cholangitis in the United Kingdom. Clin Gastroenterol Hepatol. 2019;17:1379-1387.
Hejda V, Louvet A, Civitarese A, Szczech L, Zou H, Nevens F. Results from a planned interim analysis of a randomized, double-blind, active-controlled trial evaluating the effects of obeticholic acid and bezafibrate on serum biomarkers in primary biliary cholangitis. J Hep. 2023; 78(S1):S45
Hirschfield GM, Heathcote EJ. Antimitochondria antibody-negative primary biliary cirrhosis. Clin Liver Dis. 2008;12:323-331.
Hirschfield GM, Liu X, Xu C,et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360(24):2544-2555.
Hirschfield GM, Mason A, Luketic V, et al. Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 2015;148(4):751-761.
Hirschfield GM, Dyson JK, Alexander GJM, et al. The British Society of Gastroenterology/UK-PBC primary biliary cholangitis treatment and management guidelines. Gut 2018;67(9):1568-1594.
Hirschfield GM, Bowlus CL, Mayo MJ et al. A phase 3 trial of seladelpar in primary biliary cholangitis. N Engl J Med 2024; 390(9):783-794.
Honda A, Ikegami T, Nakamuta M, et al. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology 2013;57(5):1931-1941.
Honda A, Tanaka A, Kaneko T, et al. Bezafibrate Improves GLOBE and UK-PBC scores and long-term outcomes in patients with primary biliary cholangitis. Hepatology 2019;70(6):2035-2046.
Huet PM, Deslauriers J, Tran A et al. Impact of fatigue on the quality of life of patients with primary biliary cirrhosis. Am J Gastroenterol. 2000;95:760-767.
Jahn CE, Schaefer EJ, Taam LA, et al. Lipoprotein abnormalities in primary biliary cirrhosis. Association with hepatic lipase inhibition as well as altered cholesterol esterification. Gastroenterology 1985;89(6):1266-1278.
Jones DE, Sutcliffe K, Pairman J, Wilton K, Newton JL. An integrated care pathway improves quality of life in Primary Biliary Cirrhosis. QJM 2008;101(7):535-543.
Jones DEJ, Wetten A, Barron-Millar B, et al. The relationship between disease activity and UDCA response criteria in primary biliary cholangitis: A cohort study. EBioMedicine 2022;80:104068.
Jones D, Carbone M, Invernizzi P, et al. Impact of setanaxib on quality of life outcomes in primary biliary cholangitis in a phase 2 randomized controlled trial. Hepatol Commun. 2023;7(3):e0057.
Jones DEJ, Beuers U, Bonder A, et al. Primary biliary cholangitis drug evaluation and regulatory approval: where do we go from here? Hepatology 2024; 80(5):1291-1300.
Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353:1261-1273.
Kowdley KV, Luketic V, Chapman R, et al. A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 2018;67(5):1890-1902.
Kowdley KV, Brookhart AM, Hirschfield GM, et al. Efficacy of obeticholic acid (OCA) vs placebo and external controls on clinical outcomes in primary biliary cholangitis (PBC). Am J Gastroenterol. 2024; online ahead of print.
Kowdley KV, Bowlus CL, Levy C et al. Efficacy and safety of elafibranor in primary biliary cholangitis. N Engl J Med. 2024;390(9):795-805.
Kumagi T, Guindi M, Fischer SE et al. Baseline ductopenia and treatment response predict long-term histological progression in primary biliary cirrhosis. Am J Gastroenterol. 2010;105:2186-2194.
Lammers WJ, van Buuren HR, Hirschfield GM et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: an international follow-up study. Gastroenterology 2014;147:1338-1349.
Lammers WJ, Hirschfield GM, Corpechot C et al. Development and validation of a scoring system to predict outcomes of patients with primary biliary cirrhosis receiving ursodeoxycholic acid therapy. Gastroenterology 2015;149:1804-1812.
Lammert C, Juran BD, Schlicht E, et al. Biochemical response to ursodeoxycholic acid predicts survival in a North American cohort of primary biliary cirrhosis patients. J Gastroenterol. 2014;49(10):1414-1420.
Leuschner U, Fischer H, Kurtz W et al. Ursodeoxycholic acid in primary biliary cirrhosis: results of a controlled double-blind trial. Gastroenterology 1989;97:1268-1274.
Levy C, Hejda V, Louvet A et al. Combined effect of obeticholic acid and bezafibrate in patients with primary biliary cholangitis and inadequate response to or intolerance of ursodeoxycholic acid: results from two phase 2 clinical trials. Hepatology 2023a;78 (S1):5019-C
Levy C, Kendrick S, Bowlus CL, et al. GLIMMER: A randomized phase 2b dose-ranging trial of linerixibat in primary biliary cholangitis patients with pruritus. Clin Gastroenterol Hepatol. 2023b;21(7):1902-1912.
Lindor KD, Therneau TM, Jorgensen RA, Malinchoc M, Dickson ER. Effects of ursodeoxycholic acid on survival in patients with primary biliary cirrhosis. Gastroenterology 1996;110(5):1515-1518.
Lindor KD, Bowlus CL, Boyer J, Levy C, Mayo M. Primary biliary cholangitis: 2018 practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2019;69(1):394-419.
Liu H, Liu Y, Wang L, et al. Prevalence of primary biliary cirrhosis in adults referring hospital for annual health check-up in Southern China. BMC Gastroenterol. 2010;10:100.
Lleo A, Wang GQ, Gershwin ME, Hirschfield GM. Primary biliary cholangitis. Lancet 2020;396(10266):1915-1926.
Longo M, Crosignani A, Battezzati PM, et al. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 2002;51(2):265-9.
Lu M, Li J, Haller IV; FOLD Investigators. Factors associated with prevalence and treatment of primary biliary cholangitis in United States health systems. Clin Gastroenterol Hepatol. 2018;16(8):1333-1341.
Ludwig J, Dickson ER, McDonald GS. Staging of chronic nonsuppurative destructive cholangitis (syndrome of primary biliary cirrhosis). Virchows Arch A Pathol Anat Histol. 1978;379:103-112.
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41(1):111-188.
Mang FW, Michieletti P, O'Rourke K et al. Primary biliary cirrhosis, sicca complex, and dysphagia. Dysphagia 1997;12:167-170.
Mattalia A, Quaranta S, Leung PS, et al. Characterization of antimitochondrial antibodies in health adults. Hepatology 1998;27(3):656-661.
Mayo MJ, Handem I, Saldana S, et al. Sertraline as a first-line treatment for cholestatic pruritus. Hepatology 2007;45(3):666-674.
Mells GF, Pells G, Newton JL, et al. Impact of primary biliary cirrhosis on perceived quality of life: the UK-PBC national study. Hepatology 2013;58(1):273-283.
Menon KV, Angulo P, Weston S et al. (2001) Bone disease in primary biliary cirrhosis: independent indicators and rate of progression. J Hepatol. 2001;35:316-323.
Murillo Perez CF, Hirschfield GM, Corpechot C et al. Fibrosis stage is an independent predictor of outcome in primary biliary cholangitis despite biochemical treatment response. Aliment Pharmacol Ther. 2019;50:1127-1136.
Murillo Perez CF, Harms MH, Lindor KD et al. Goals of treatment for improved survival in primary biliary cholangitis: Treatment target should be bilirubin within the normal range and normalization of alkaline phosphatase. Am J Gastroenterol. 2020;115(7):1066-1074.
Murillo Perez CF, Fisher H, Hiu S, et al. Greater transplant-free survival in patients receiving obeticholic acid for primary biliary cholangitis in a clinical trial setting compared to real-world external controls. Gastroenterology 2022;163(6):1630-1642.
Nakanuma Y, Zen Y, Harada K et al. Application of a new histological staging and grading system for primary biliary cirrhosis to liver biopsy specimens: Interobserver agreement. Pathol Int. 2010;60:167-174.
Nakamura M, Kondo H, Mori T et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology 2007;45:118-127.
Natarajan Y, Tansel A, Patel P et al. Incidence of Hepatocellular carcinoma in primary biliary cholangitis: A systematic review and meta-analysis. Dig Dis Sci. 2021;66:2439-2451.
Nevens F, Andreone P, Mazzella G, et al. A Placebo-Controlled Trial of Obeticholic Acid in Primary Biliary Cholangitis. N Engl J Med. 2016;375(7):631-643.
Nevens F, Shiffmann ML, Drenth JPH et al. Durable response in the markers of cholestasis through 5 years of open-label extension study of obeticholic acid in primary biliary cholangitis. Hepatology 2019; 70:S1:LB06
Nevens F, Trauner M, Manns MP. Primary biliary cholangitis as a roadmap for the development of novel treatments for cholestatic liver diseases. J Hepatol. 2023;78(2):430-441.
Osman KT, Maselli DB, Idilman IS et al. Liver stiffness measured by either magnetic resonance or transient elastography is associated with liver fibrosis and is an independent predictor of outcomes among patients with primary biliary cholangitis. J Clin Gastroenterol. 2021;55:449-457.
Pares A, Caballeria L, Rodes J. Excellent long-term survival in patients with primary biliary cirrhosis and biochemical response to ursodeoxycholic Acid. Gastroenterology 2006;130:715-720.
Poupon RE, Balkau B, Eschwège E, Poupon R. A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N Engl J Med. 1991;324(22):1548-1554.
Poupon RE, Poupon R, Balkau B. Ursodiol for the long-term treatment of primary biliary cirrhosis. The UDCA-PBC Study Group. New Engl J Med. 1994;330(19):1342-1347.
Poupon RE, Lindor KD, Cauch-Dudek K, Dickson ER, Poupon R, Heathcote EJ. Combined analysis of randomized controlled trials of ursodeoxycholic acid in primary biliary cirrhosis. Gastroenterology 1997; 113:884-890.
Poupon RE, Chretien Y, Chazouilleres O et al. Quality of life in patients with primary biliary cirrhosis. Hepatology 2004;40:489-494.
Prince MI, Chetwynd A, Craig WL, Metcalf JV, James OF. Asymptomatic primary biliary cirrhosis: clinical features, prognosis, and symptom progression in a large population based cohort. Gut 2004;53(6):865-870.
Reig A, Sesé P, Parés A. Effects of Bezafibrate on Outcome and Pruritus in Primary Biliary Cholangitis With Suboptimal Ursodeoxycholic Acid Response. Am J Gastroenterol. 2018;113(1):49-55.
Reig A, Álvarez-Navascués C, Vergara M, et al. Obeticholic acid and fibrates in primary biliary cholangitis: Comparative effects in a multicentric observational study. Am J Gastroenterol. 2021;116(11):2250-2257
Roberts SB, Ismail M, Kanagalingam G, et al. Real-World Effectiveness of Obeticholic Acid in Patients with Primary Biliary Cholangitis. Hepatol Commun. 2020;4(9):1332-1345.
Roll J, Boyer JL, Barry D, Klatskin G. The prognostic importance of clinical and histologic features in asymptomatic and symptomatic primary biliary cirrhosis. N Engl J Med. 1983; 6;308(1):1-7.
Scheuer PJ. Ludwig Symposium on biliary disorders--part II. Pathologic features and evolution of primary biliary cirrhosis and primary sclerosing cholangitis. Mayo Clin Proc. 1998;73:179-183.
Selmi C, Bowlus CL, Gershwin ME, Coppel RL. Primary biliary cirrhosis. Lancet 2011;377(9777):1600-1609.
Silveira MG, Suzuki A, Lindor KD. Surveillance for hepatocellular carcinoma in patients with primary biliary cirrhosis. Hepatology 2008;48(4):1149-56.
Silveira MG, Gossard AA, Stahler AC, et al. A randomized, placebo-controlled clinical trial of efficacy and safety: Modafinil in the treatment of fatigue in patients with primary biliary cirrhosis. Am J Ther. 2017;24(2):e167-e176.
Smets L, Verbeek J, Korf H, van der Merwe S, Nevens F. Improved Markers of Cholestatic Liver Injury in Patients With Primary Biliary Cholangitis Treated With Obeticholic Acid and Bezafibrate. Hepatology 2021;73(6):2598-2600.
Smith H, McLaughlin MM, Das S et al. The devastating impact of severe pruritus in primary biliary cholangitis; Hepatol Comm. 2025; online ahead of print.
Soret PA, Lam L, Carrat F, et al. Combination of fibrates with obeticholic acid is able to normalise biochemical liver tests in patients with difficult-to-treat primary biliary cholangitis. Aliment Pharmacol Ther 2021;53(10):1138-1146.
Ständer S, Augustin M, Reich A, et al. Pruritus assessment in clinical trials: consensus recommendations from the International Forum for the Study of Itch (IFSI) Special Interest Group Scoring Itch in Clinical Trials. Acta Derm Venereol. 2013;93(5):509-514.
Ständer S, Zeidler C, Augustin M, et al. S2k guideline: Diagnosis and treatment of chronic pruritus. J Dtsch Dermatol Ges. 2022;20(10):1387-1402.
Tajiri K, Shimizu Y. Recent advances in the management of pruritus in chronic liver diseases. World J Gastroenterol. 2017;23(19):3418-3426.
Tanaka A, Hirohara J, Nakanuma Y, Tsubouchi H, Takikawa H. Biochemical responses to bezafibrate improve long-term outcome in asymptomatic patients with primary biliary cirrhosis refractory to UDCA. J Gastroenterol. 2015;50(6):675-682.
Tanaka A, Hirohara J, Nakano T, et al. Association of bezafibrate with transplant-free survival in patients with primary biliary cholangitis. J Hepatol. 2021;75(3):565-571.
Tandon P, Rowe BH, Vandermeer B, Bain VG. The efficacy and safety of bile Acid binding agents, opioid antagonists, or rifampin in the treatment of cholestasis-associated pruritus. Am J Gastroenterol. 2007;102(7):1528-1536.
Trauner M, Nevens F, Shiffman ML, et al. Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet Gastroenterol Hepatol. 2019;4(6):445-453.
Trivedi PJ, Lammers WJ, van Buuren HR et al. Stratification of hepatocellular carcinoma risk in primary biliary cirrhosis: a multicentre international study. Gut 2016;65:321-329.
Untas A, Boujut E, Corpechot C, et al. Quality of life and illness perception in primary biliary cirrhosis: a controlled cross-sectional study. Clin Res Hepatol Gastroenterol 2015;39(1):52-58.
Vergani D, Alvarez F, Bianchi FB, et al. Liver autoimmune serology: a consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J Hepatol. 2004;41(4):677-83.
Vleggaar FP, van Buuren HR, Zondervan PE et al. Jaundice in non-cirrhotic primary biliary cirrhosis: the premature ductopenic variant. Gut 2001;49:276-281.
Webb GJ, Rahman SR, Levy C, Hirschfield GM. Low risk of hepatotoxicity from rifampicin when used for cholestatic pruritus: a cross-disease cohort study. Aliment Pharmacol Ther. 2018;47(8):1213-1219.
Yagi M, Tanaka A, Namisaki T, et al. Is patient-reported outcome improved by nalfurafine hydrochloride in patients with primary biliary cholangitis and refractory pruritus? A post-marketing, single-arm, prospective study. J Gastroenterol. 2018;53(10):1151-1158.
Younossi ZM, Bernstein D, Shiffman ML, et al. Diagnosis and management of primary biliary cholangitis. Am J Gastroenterol. 2019;114(1):48-63.
Zandanell S, Strasser M, Feldman A, et al. Low rate of new-onset primary biliary cholangitis in a cohort of anti-mitochondrial antibody-positive subjects over six years of follow-up. J Intern Med. 2020;287(4):395-404.
