9. MASLD / MASH
Andreas Geier, Elke Roeb
Summary
Metabolic dysfunction-associated steatotic liver disease (MASLD) formerly known as non-alcoholic fatty liver disease (NAFLD) is viewed as a serious health concern in industrial countries. MASLD pathogenesis and its detailed progression to fibrosis and chronic liver disease is still unclear. Many studies have shown that MASLD/NAFLD may be associated with increased insulin resistance (IR). IR, obesity, low adiponectin, (postprandial) dyslipidaemia, and hyperglycaemia represent the main factors leading to MASLD and accelerate the course and progression of this disease. MASLD can affect people of all ages and appears to vary in different ethnic groups. Environmental and lifestyle factors such as reduced physical activity and high-fat diets are well-studied factors in the development of IR-associated comorbidities and MASLD. Recent studies have made advances in the area of genetic risk factors and immune responses in metabolic dysfunction-associated steatohepatitis (MASH) pathogenesis. Changing lifestyle in form of weight loss, dietary changes and physical activity is an important therapeutic measure. Basic drug therapy is based on the associated diseases (dyslipidaemia, obesity, diabetes). Specific approved MASLD drugs for advanced liver fibrosis (F2-3) include Resmetirom (EMA-, FDA-) and Semaglutide (FDA). In case of severe obesity, bariatric surgery might be performed to treat MASLD and MASH. In case of severe MASH-complications liver transplantation might be an option. Targeted interventions in the numerous mechanisms involved in the progression of MASH are intended in particular to prevent the development and progression of liver fibrosis. Follow-up and surveillance of MASH patients is recommended according to their individual risk.
The new nomenclature for fatty liver disease used in this chapter is based on an international consensus from the hepatological societies AASLD (USA) and EASL (Europe), which the German DGVS has also explicitly endorsed. [Rinella 2023] The new definition requires the presence of at least one cardiometabolic risk factor in addition to hepatic steatosis. The term metabolic dysfunction-associated steatotic liver disease (MASLD) was defined as a replacement term for NAFLD and metabolic dysfunction-associated steatohepatitis (MASH) as a replacement term for NASH. The acronym MetALD was chosen to designate a separate group of patients with MASLD who consume 140-350 g/week in women and 210-420 g/week in men. The proposed nomenclature allows for flexible refinement as new insights into the underlying pathophysiology and risk factors of hepatic steatosis are gained. An analysis by the European LITMUS consortium showed that 98% of the existing registry cohort of patients with NAFLD would fulfil the new criteria for MASLD.(Hardy, Wonders et al. 2020, Rinella, Lazarus et al. 2023) In the North American NHANES cohort and the national Swedish registry, there is even a 99% match between the diagnoses of MASLD and NAFLD.(Hagström, Vessby et al. 2023, Lee, Dodge et al. 2023) In places where NASH was used as a histological diagnosis, this is indicated by the use of both terms (MASH/NASH).
Introduction
According to the current guidelines of the DGVS (German Society for Gastroenterology Digestive and Metabolic Diseases) (Roeb, Canbay et al. 2022), EASL (European Association for the Study of the Liver) (2016, EASL-EASD-EASO 2024), AASLD (American Association for the Study of Liver Diseases) (Chalasani, Younossi et al. 2018, Chen, Morgan et al. 2025), APASL (Asian Pacific Association for the Study of the Liver, HCC Guideline, 2017) (Kim, Lee et al. 2017), and the World Gastroenterology Organization (2012) (LaBrecque, Abbas et al. 2014) Metabolic dysfunction-associated steatotic liver disease (MASLD) includes the fatty liver diseases MASL (formerly non-alcoholic fatty liver), MASH (metabolic dysfunction associated steatohepatitis), MASH fibrosis and MASH cirrhosis. Other nomenclatures (e.g. metabolically associated fatty liver disease or metabolic dysfunction-associated fatty liver disease / MAFLD) have been proposed to strengthen the relevance of metabolic disorders in this context.(Roeb 2021)
The progression of MASH is associated with liver cell stress, consecutive inflammation and fibrosis, with potential development of liver cirrhosis, portal hypertension and end-stage liver disease. MASH is also a relevant risk factor for the occurrence of hepatocellular carcinoma (HCC). The pathogenesis and natural course of MASLD are increasingly better understood, even if the heterogeneity of the patients and the multifactorial triggers make it difficult to estimate the individual prognosis. End-stage MASH-associated liver disease is expected to represent the highest proportion of patients listed for liver transplantation in the future. Although genetic factors have also been identified, the disease is thought to be primarily a consequence of hyperalimentation and the hepatic manifestation of the metabolic syndrome.(Loomba, Friedman et al. 2021) The clinical symptoms of non-cirrhotic MASLD are usually non-specific.(Geier, Rinella et al. 2021) With a global prevalence of approximately 25%, MASLD is now the leading cause of chronic liver disease worldwide and a growing public health challenge. A further increase in MASLD in the sense of the obesity epidemic, especially among adolescents and younger patients, is to be expected. Changes in lifestyle and demographic changes are causing an increase in MASLD prevalence. Doctors and patient organisations have to deal with this collectively and individually.(Roeb, Canbay et al. 2022) In 2023, a new category was introduced alongside pure metabolic dysfunction-associated steatotic liver disease, called metabolic and alcohol-related/associated liver disease (MetALD). This category describes people with metabolic dysfunction-associated steatotic liver disease who consume larger amounts of alcohol per week (140-350 g/week and 210-420 g/week for women and men respectively).(Rinella, Lazarus et al. 2023)
Prevalence
Metabolic dysfunction-associated steatotic liver disease (MASLD) has become a major disease burden in the last decades. The estimated overall global prevalence of MASLD in the general population diagnosed by imaging is about 25%-30% with an expected significant increase in the next years.(Younossi, Koenig et al. 2016, Estes, Anstee et al. 2018, Younossi, Golabi et al. 2023) Newest data report a global MASLD prevalence of 30% and increasing in adults, and 7,4% in children/adolescents which requires urgent and comprehensive strategies on local, regional, and global levels.(Paik, Henry et al. 2023)
The incidence of MASLD ranges globally between 28 and 52 per 1000 person years.(Younossi, Henry et al. 2018, Paik, Henry et al. 2023) In absolute numbers, it is estimated that 64 million subjects are affected by MASLD in the United States and 52 million in Europe.(Younossi, Koenig et al. 2016) Following a steady increase over the past decades, MASLD represents in the meantime the second most common indication for liver transplantation in the north American UNOS network.(Younossi, Stepanova et al. 2021) On the European transplant waiting list by far less end-stage patients with MASLD appear in the ELTR registry.(Haldar, Kern et al. 2019)
Global estimates of MASLD prevalence vary among the different continents and range from 32% in the Middle East and 31% in South America to 23% in Europe, 24% in the United States and 27% in Asia.(Younossi, Koenig et al. 2016, Younossi, Henry et al. 2018) The steady increase over the past decades can be monitored globally. According to recent modelling, the number of MASLD patients in Germany has been estimated to be 18.4 million with more than 3 million affected by MASH. The number of MASH patients with advanced fibrosis may be as high as 600.000 and is expected to more than double until 2030.(Estes, Anstee et al. 2018) Projections suggest that the number of NASH cases in the United States will increase by 82.6% from 11.61 million (2020) to 19.53 million (2039).(Younossi, Paik et al. 2023)
In a population based study with data from 2007-2012, the prevalence of FIB-4 >2.67 was 1.1% in the general population which is in rough accordance with these numbers.(Huber, Schulz et al. 2022) Given an estimated current number of 200.000 MASH patients with cirrhosis in Germany, it appears remarkable that MASLD/MASH represented only 13% of the indications on German liver transplantation waiting lists in 2015.(Tacke, Kroy et al. 2016) One reason for this discrepancy may be the frequently absent diagnosis in clinical reality. It has been suggested that MASH accounts for more than 50% of cases of cryptogenic cirrhosis.(Ratziu, Giral et al. 2000)
Among all subjects with MASLD, the relative proportion of MASH as the inflammatory and progressive disease entity ranges from 10% to 20%. The absolute prevalence of MASH in Western countries is approximately 2-6%.(Younossi, Koenig et al. 2016, Younossi, Henry et al. 2018) Due to a preselection of patients at risk, the prevalence of MASH/NASH in liver biopsies of NAFLD/MASLD patients is around 60-70%.(Younossi, Koenig et al. 2016)
Demographics and risk factors
MASLD is more prevalent in males than in females and its prevalence increases with age.(Roeb, Canbay et al. 2022, Le, Le et al. 2023) There is a clear association with metabolic comorbidities. In German MASLD cohorts, more than 30-60% of MASLD patients have type 2 diabetes, 37-84% show hyperlipidaemia, and 52-67% have arterial hypertension.(Labenz, Huber et al. 2018, Alsenbesy, Rau et al. 2019, Hofmann, Buggisch et al. 2020, Geier, Rau et al. 2023) All comorbidities were more prevalent in high risk as compared to low risk patients (FIB-4 <1.3) including arterial hypertension (85% vs. 42%), hypercholesterolaemia (39% vs. 16%), and type 2 diabetes mellitus (69% vs. 26%).(Geier, Rau et al. 2023)
Increased BMI, particularly visceral obesity and presence of the metabolic syndrome are established risk factors for the presence of MASLD. The prevalence of MASLD has been continuously rising over the past decades along the global increase in BMI and obesity, which affects more than 650 million subjects worldwide.(Younossi, Loomba et al. 2018) Based on ultrasound data, the prevalence of MASLD increases proportionally to BMI from 25% with normal BMI up to >90% in subjects with obesity (BMI >30kg/m2).(Bedogni, Miglioli et al. 2007) The prevalence of type 2 diabetes has increased in parallel to the increasing prevalence of obesity with more than 400 million affected subjects worldwide.(Younossi 2019) MASLD affects 60-70% of patients with type 2 diabetes and the presence of diabetes represents a significant risk factor for fibrosis progression and cirrhosis development.(Younossi, Koenig et al. 2016, Younossi, Anstee et al. 2018) Based on the steady increase in diabetes over the past, projections for the year 2030 estimate an increase by around 50% in MASH and even a more than doubling in MASH patients with end-stage liver disease.(Estes, Anstee et al. 2018) The expected increase in end-stage disease is at least partly explained by the aging of western populations. Prevalence and advanced stages of the disease are both increasing with patient age, which in turn is associated with more frequent metabolic comorbidities.(Younossi 2019, Roeb, Canbay et al. 2022)
MASLD represents a multifactorial disease caused by environmental, genetic, and epigenetic factors. The inheritable component accounts for 20-40% of the NAFLD phenotype.(Zimmer and Lammert 2011) The prevalence of MASLD differs with ethnicity and is highest in subjects with Hispanic descent.(Williams, Stengel et al. 2011, Rich, Oji et al. 2018) Several single nucleotide polymorphisms have been associated with an increased prevalence of MASLD. The most relevant has been located in the patatin‐like phospholipase domain‐containing protein 3 (PNPLA3) gene in the first genome-wide association study.(Romeo, Kozlitina et al. 2008) Indeed, the PNPLA3 risk G allele frequency worldwide is highest in populations of Mesoamerica.
However, around 20% of patients with MASLD have normal body weight and were therefore formerly referred to as lean NAFLD (BMI <25kg/m² or <23kg/m² in Asians).(Ye, Zou et al. 2020, Young, Tariq et al. 2020) Lean NAFLD patients have a different disease phenotype with less inflammatory activity, increased odds for genetic contribution such as PNPLA3 risk allele polymorphism and probably a better prognosis.(Ye, Zou et al. 2020, Young, Tariq et al. 2020) In non-obese MASLD the PNPLA3 risk allele frequency exceeds the prevalence of obese MASLD patients with almost 80% carrying at least one risk allele.(Krawczyk, Liebe et al. 2020) In the new internationally agreed nomenclature, the term lean NAFLD no longer appears and is included in the group of MASLD patients.(Rinella, Lazarus et al. 2023)
Pathogenesis
Hepatic steatosis (fatty liver cells) is characterised by storage of fat in more than 5% of hepatocytes. Steatohepatitis is present when inflammation and liver cell damage can be detected in addition to hepatic steatosis.(Loomba, Friedman et al. 2021) Although diet-related and alcoholic causes of steatosis and steatohepatitis are the most common, the differential diagnostic spectrum of possible causes of obesity-associated liver damage is broad. These causes should be determined in any case and considered for the final interpretation of the liver damage.
Both, alcoholic (ASH) and non-alcoholic steatohepatitis (MASH/NASH) are characterised by fatty degeneration and lobular inflammation with ballooning of liver cells, resulting in mesh wire fibrosis (progressive if the inflammation persists).(Kleiner, Brunt et al. 2005) In general, a reliable differential diagnosis of ASH vs. MASH/NASH cannot be made solely on the basis of histological criteria. The differences between ALD and MASLD worked out are of a gradual nature and therefore not sufficiently reliable for the typing of the individual case (cave: lifestyle modification before the liver biopsy). Fatty degeneration and the formation of glycogen hole cores are often more pronounced in MASH/NASH, while inflammatory activity and the detection of Mallory Denk Bodies (MDB) and satellitosis (granulocytic demarcation of a hepatocyte with MDB) might be observed more frequently in ASH.(Morita, Ueno et al. 2005)
Most patients with MASLD have central obesity and other components of a metabolic syndrome. However, MASLD can also develop in patients of normal weight (formerly lean NAFLD, approx. 20% of cases, BMI = 18.5-24.9 kg/m2). It is assumed that these patients may have less inflammatory activity and therefore have a better prognosis.(Ye, Zou et al. 2020) Due to the frequent association with the metabolic syndrome, a consensus panel suggested that NAFLD could be called metabolic-associated fatty liver disease (MAFLD).(Eslam, Sanyal et al. 2020) This designation, however, excludes some entities. On the one hand, the lean NAFLD in the absence of metabolic comorbidities is blurred; on the other hand, the congenital metabolic diseases (e.g. mitochondriopathies, glycogenoses) represent independent pathogenetic and therapeutic entities.(George, Gish et al. 2021) The new nomenclature means that all fatty liver diseases, including ALD, monogenetic and cryptogenic liver damage and drug-induced liver injury, are now summarised under the term steatotic liver disease, SLD.(Rinella, Lazarus et al. 2023)
Figure 1 depicts the positive and negative factors for the development of MASLD. The diagnosis of MASH represents a precancerous condition/lesion, so that it can lead to the development of hepatocellular carcinoma (HCC) and more rarely intrahepatic cholangiocarcinoma (iCCA; ratio: 5-7 HCC/ 1 iCCA). As detailed below, tumour surveillance should be performed according to current liver cancer guidelines.(Loomba, Lim et al. 2020)
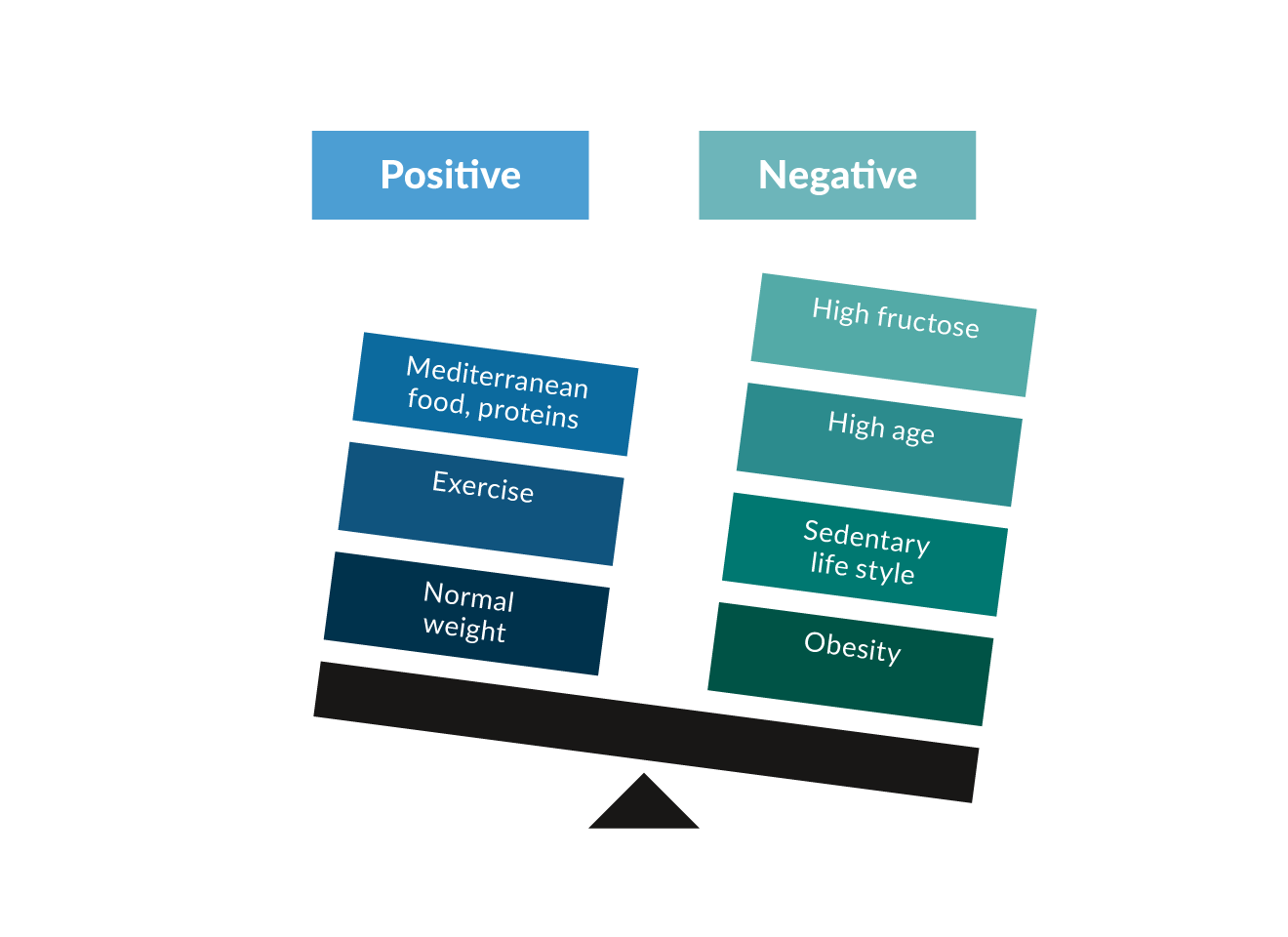 Figure 1. Risk factors (negative) and protective factors (positive) for the development of MASLD.
Figure 1. Risk factors (negative) and protective factors (positive) for the development of MASLD.
The decisive factor for the prognosis of MASLD patients is the stage of fibrosis. A meta-analysis from five studies involving 1495 biopsy-proven MASLD/NAFLD patients and a follow-up of 17,452 patient-years showed that compared to MASLD patients without fibrosis (F0), those with fibrosis were at increased risk for both, total and also liver-specific mortality, which increased continuously with the fibrosis stage. With regard to liver-specific mortality, an exponential increase in risk was recorded.(Dulai, Singh et al. 2017) The greatest risk for liver-specific but also overall morbidity and mortality from MASLD was found for advanced fibrosis (F3) and liver cirrhosis (F4). The following event rates existed in an average observation period of 5.5 years: 8% all-cause mortality, 8% liver transplantation, 19% first-time hepatic decompensation, 9% HCC, 3% vascular events and 7% non-hepatic malignancies. The 10-year transplant-free survival was 94% for F3 and 45.5% for F4. Higher cumulative incidences of vascular events (7% vs. 2%) and non-hepatic malignancies (14% vs. 6%) were found in F3. In patients with liver cirrhosis, on the other hand, the frequency of hepatic decompensation and HCC development was increased: 44% (F4) vs. 6% (F3) and 17% (F4) vs. 2.3% (F3).(Vilar-Gomez, Calzadilla-Bertot et al. 2018) These data suggest that cardiovascular and non-hepatic morbidity and mortality are already dominant in non-cirrhotic MASLD patients, while the complications of advanced liver disease determine further prognosis in established liver cirrhosis.
Xiao et al. conducted a meta-analysis of over 13,000 subjects to determine the best method for assessing fibrosis in MASLD. Comparing APRI, FIB-4, BARD score, NAFLD fibrosis score (NFS), transient hepatic elastography, shear wave elastography (SWE) and magnetic resonance elastography (MRE), MRE and SWE showed the highest diagnostic accuracy for the fibrosis stage. Among the four non-invasive simple indices, NFS and FIB-4 were at best for detecting advanced fibrosis.(Xiao, Zhu et al. 2017) According to current meta-analyses, complex biomarker panels and elastography can identify MASLD-related fibrosis with moderate accuracy in obese subjects, but these methods are not yet well validated.(Ooi, Mgaieth et al. 2018)
Human genetic factors
Genetic factors play a considerable role in the development of hepatic fat accumulation. In 2008, two GWAS studies linked the rs738409 polymorphism (I148M) of PNPLA3 (see above) with hepatic fat content and alanine aminotransferase (ALT) levels.(Romeo, Kozlitina et al. 2008, Yuan, Waterworth et al. 2008) Further studies suggest that the I148M variant is an important risk factor for the development of MASH fibrosis, cirrhosis and hepatocellular carcinoma.(Dubuquoy, Burnol et al. 2013) The I148M PNPLA3 variant favours hepatic carcinogenesis not only in steatohepatitis but also in other liver diseases. Further GWAS studies have uncovered robust and reproducible associations in other genes including transmembrane 6 superfamily member 2 (TM6SF2), membrane bound O-acyltransferase domain-containing 7 (MBOAT7) and more recently in the 17-beta hydroxysteroid dehydrogenase 13 (HSD17B13) genes.(Trépo and Valenti 2020) Phenome-wide association studies (PheWAS) for disease endpoints revealed that PNPLA3 is also associated to other forms of liver injury and support the hypothesis that therapeutic inhibition of PNPLA3 could treat liver diseases.(Diogo, Tian et al. 2018) At this time, the use genetic SNPs as biomarkers for risk assessment are not recommended in clinical routine since the odds ratio of these variants to predict clinical endpoints is too low to justify their use.(Roeb, Canbay et al. 2022) However, combined models may be used in the future. Combined effects of PNPLA3, TM6SF2 and MBOAT7 have been detected on liver damage in MASLD as transaminase levels are proportionally increasing with increasing number of risk alleles present.(Krawczyk, Rau et al. 2017)
Microbiome
Several studies indicate that the intestinal microbiome is involved in both the development and progression of MASLD.(Boursier, Mueller et al. 2016) NASH patients are characterised, for example, by a different composition of the gut microbiome with higher faecal levels of short-chain fatty acids (SCFAs) and an increased frequency of SCFAs-producing bacteria. These changes are associated with immunological features of MASLD progression.(Rau, Rehman et al. 2018) However, no specific microbiota composition for MASLD can currently be determined. Therefore, stool diagnostics are currently not suitable for screening or diagnosing MASLD.
Fetal development is influenced not only by genetics, but also by the interaction of parental and environmental conditions before conception and during pregnancy. Collectively, these factors could lead to fetal programming of adulthood diseases, including MASLD. Programming of MASLD can be influenced by maternal factors such as obesity/overweight, nutritional status, health status, changes in the microbiome and epigenetic changes, vitamin supplements, use of medications during pregnancy, and exposure to environmental pollutants. Paternal factors such as nutritional status, exercise, and alcohol consumption may also play important roles in programming for MASLD.(Galvan-Martinez, Bosquez-Mendoza et al. 2023)
Natural history
So far, histological MASH/NASH has been considered the progressive form of MASLD; meanwhile it has been repeatedly shown that simple steatosis can also be progressive.(Sanyal, Harrison et al. 2019) In a meta-analysis of 11 studies with paired biopsies, fibrosis progression by one stage was 14.3 years for NAFL and 7.1 years for MASH/NASH.(Singh, Allen et al. 2015) In another large study (n=646), the mean time to the development of end-stage liver cirrhosis was examined in biopsy-confirmed MASLD and an observation period of 20 years. Time to cirrhosis was for F0; F1; F2; F3 and F4 each at 33.4; 34.1; 22.7; 11.8 and 5.6 years. As outlined, the decisive factor for MASLD prognosis is the underlying stage of fibrosis.(Dulai, Singh et al. 2017) The greatest risk for liver-specific but also overall morbidity and mortality in NAFLD is the presence of advanced fibrosis (F3) and liver cirrhosis (F4). Cardiovascular and non-hepatic morbidity and mortality are the main factors in non-cirrhotic MASLD patients (Long, Zhang et al. 2021), while the complications of advanced liver disease determine the further prognosis in patients with overt liver cirrhosis. The latter particularly includes the risk of HCC development. Depending on the region and study population, the prevalence is between 0.8% and 34%.(Younossi 2019) The major challenge is that in MASLD, HCC can also develop in non-cirrhotic livers (up to 20-50% of cases in some series).(Kanwal, Kramer et al. 2018) Following epidemiology, MASLD is increasingly becoming an indication for LTX (see below).
Depending on the fibrosis stage, patients with MASLD have increased liver-related and all-cause mortality compared to healthy controls. Cardiovascular causes of death come first. In a retrospective analysis of 619 NAFLD patients over the period 1975-2005 and a median follow-up of 12.6 years, cardiovascular disease was the most common cause of death (38%), followed by non-hepatic cancer (19%) and complications of liver cirrhosis (8%).(Angulo, Kleiner et al. 2015) Similar data come from two prospective studies from Sweden with a follow-up up to 33 years: cardiovascular causes of death 43% and 48%, non-hepatic tumours 23% and 22%, and liver-related mortality 9% and 10%.(Ekstedt, Hagström et al. 2015, Nasr, Ignatova et al. 2018) A meta-analysis with 6,263 patients showed that MASLD is associated with the occurrence of extrahepatic tumours such as colorectal adenomas (OR 1.74).(Shen, Lipka et al. 2014) In a study with 25,497 participants and an observation period of 7.5 years, patients with MASLD showed an increased incidence of colorectal carcinoma in men and breast carcinoma in women in addition to the known risk of HCC, especially in advanced fibrosis.(Kim, Lee et al. 2017, Yang, Teng et al. 2023) Surveillance of these patients is therefore warranted.
Screening
Liver fibrosis is the only liver lesion independently associated with long-term overall mortality, liver transplantation, and liver-related events in MASLD.(Angulo, Kleiner et al. 2015, Dulai, Singh et al. 2017) Aim of the screening process is therefore to identify patients with progressive fibrosis who are at risk to develop complications over time. Several non-invasive tests (NIT) are available for the evaluation of liver fibrosis in MASLD, essentially blood-based tests and elastography devices including Fibroscan (vibration controlled transient elastography, VCTE).(Loomba and Adams 2020) Investigating a larger region of interest compared to the volume of a liver biopsy, elastography procedures offer obvious advantages and have a good accuracy for the diagnosis of advanced F3-4 fibrosis. In a recent individual patient data meta-analysis AUROCs of individual VCTE, Fibrosis-4 Index (FIB-4) and NAFLD Fibrosis Score (NFS) for advanced fibrosis were 0.85, 0.76 and 0.73.(Mózes, Lee et al. 2022) Both, blood-based tests and elastography can identify the subset of MASLD patients who have an impaired liver-related prognosis and who therefore require an intensive management of their liver disease.
According to current guidelines, screening is recommended in the established populations with an increased risk for fatty liver disease but should be performed in the unselected general population.(2016, Chalasani, Younossi et al. 2018, Roeb, Canbay et al. 2022) As outlined above, these populations at risk are subjects with obesity, metabolic syndrome or metabolic disease such as type 2 diabetes, hypertension, hyperlipidaemia. In current real world settings, the initial diagnosis of MASLD is typically achieved through suggestive laboratory results and ultrasound findings mostly in patients with obesity and/or metabolic risk.(Roeb, Canbay et al. 2022) Principal aim of the initial workup of patients with MASLD is the identification of patients at risk for advanced fibrosis and in turn related clinical events over time. Even more important is the exclusion of advanced disease in low-risk patients who will not be considered for further costly and time-consuming workup. Based on clinical risk factors for disease progression such as age, presence of diabetes and/or laboratory findings, various algorithms have been established for initial MASLD risk assessment (NAFLD Fibrosis Score (NFS) and fibrosis-4 index (FIB-4)) and are recommended in current guidelines.(2016, Chalasani, Younossi et al. 2018, Roeb, Canbay et al. 2022) Thresholds for the different risk categories mentioned in table 1 are as follows: FIB-4 score (<1.3, ≥1.3-<2.67, ≥2.67 for low, indeterminate and high risk), NAFLD fibrosis score (NFS) (<-1.455, -1.455-0.676, >0.676 for low, indeterminate and high risk). For patients with intermediate or high risk in primary testing (≤8.0 kPa, >8.0-<10.0 kPa, ≥10.0 kPa for low, indeterminate and high risk), elastography is recommended in a second step to guide the decision for liver biopsy to confirm advanced hepatic fibrosis. Again, patients with low risk of advanced disease will not be considered for further workup. Primary goal of the proposed algorithms is to narrow down the large number of patients with putative MASLD (mostly suggestive ultrasound findings) in primary care (estimated 25-30%) to a limited number of patients at risk for secondary and tertiary care (estimated 3-5%).(Dietrich, Rau et al. 2021) In contrast to fibrosis assessment, no NIT has so far achieved enough accuracy and validation for the non-invasive diagnosis of MASH.
Diagnosis
MASLD has been defined as a liver disease with more than 5% steatotic hepatocytes in the absence of a relevant alcohol consumption and secondary causes of hepatic lipid accumulation. Since only approximately 10% of Western populations are completely abstinent from alcohol, thresholds of a daily alcohol ingestion of 10-20 g in females and 20-30 g in males (different thresholds in the international guidelines) have been defined to differentiate “MASLD “ from metALD and ALD.(Roeb, Canbay et al. 2022, Rinella, Lazarus et al. 2023)
The initial diagnosis of MASLD is most frequently made by routine ultrasound based on typical finding of a “bright“ or “hyperechogenic” liver parenchyma. Of note, the sensitivity of ultrasound is only sufficient to reliably detect hepatic steatosis of 30% or more.(Saadeh, Younossi et al. 2002) Hepatic steatosis can be semi-quantitatively graded into four grades (0-3). Integrated into the FibroScan® device, controlled attenuation parameter (CAP) has been developed as a more sensitive non-invasive method for detection and quantification of hepatic steatosis. CAP detects the attenuation of the ultrasound which correlates with the hepatic fat content and is given in dB/m (reference value for absent steatosis <250 dB/m). The AUROC for the detection of nay hepatic steatosis is 0.82.(Karlas, Petroff et al. 2017) The most obvious advantage of CAP over ultrasound is the high sensitivity with a lower treshold of 5% for fat detection. At least as sensitive as CAP is the magnetic resonance (MR) based fat quantification which represents the gold standard. MR Proton density fat fraction (PDFF) has emerged to be a promising tool in precise fat quantification with a lower detection limit of 5%.(Loomba 2018) However, this method is not widely available at present and mostly used for clinical research in academic centres.
The diagnosis of MASH is restricted to histopathological workup as this disorder is characterised by the simultaneous presence of steatosis, hepatocyte damage, lobular inflammation and fibrosis with centrilobular (zone 3) pattern of injury. The term “fatty liver hepatitis” as a surrogate of “steatohepatitis” first appeared in 1962 in the German literature to describe fatty liver with necroinflammation.(Geier, Tiniakos et al. 2021) Subsequently, Jurgen Ludwig from the Mayo Clinic, Rochester, MN, USA coined the term “non-alcoholic steatohepatitis” in 1980 as a “hitherto unnamed liver disease that histologically mimics alcoholic hepatitis and that also may progress to cirrhosis”.(Ludwig, Viggiano et al. 1980) The NASH Clinical Research Network (NASH CRN) established and validated the NASH CRN score as the first globally accepted, scoring system that addressed the full spectrum of MASLD lesions and proposed the summative NAFLD activity score (NAS) to semi-quantify disease activity in clinical trials.(Kleiner, Brunt et al. 2005) The NAS (range 0-8) is calculated by summing-up semi-quantitative scores for three of the most important histological features of MASLD: steatosis (0-3), lobular inflammation (0-2), and hepatocellular ballooning (0-2). Kleiner and colleagues observed that NAS >5 correlated with MASH diagnosis whereas biopsies with NAS scores of <3 correlated with “not MASH.”(Kleiner, Brunt et al. 2005) However, MASLD displays a continuous spectrum of hepatocytic, inflammatory and fibrous lesions and therefore, the binary categorisation of MASLD into MASH and “not MASH/NASH” is artificial in a continuous disease process.(Bedossa 2013) In 2012, Bedossa and colleagues developed a simple algorithm to standardise the histological diagnosis of MASH and reduce inter-observer variability. This diagnostic algorithm was informed by scores for steatosis (S0-S3), activity grade (A0-A4 by adding scores for ballooning (0-2) and lobular inflammation (0-2)) and fibrosis stage (F0-F4).(Bedossa, Poitou et al. 2012) Validated by pathologists from the Fatty Liver Inhibition of Progression (FLIP) consortium, the SAF scoring system (Steatosis, Activity, Fibrosis) includes the same categories as NAS for the semi-quantitation of liver injury but the diagnostic FLIP algorithm requires the simultaneous presence of steatosis, ballooning and lobular inflammation for MASH diagnosis.(Bedossa and Consortium 2014)
The available elastography devices calibrated on the different fibrosis stages allow of fully non-invasive stratification of MASLD that is well adapted to decision-making in clinical practice. However, the sensitivity and specificity for early fibrosis stages is modest and a high rate of false positive results limit the positive predictive value of these methods.
Recently, the LiverRisk score was prospectively developed from an international cohort from six countries of individuals in the general population without known liver disease who underwent assessment of liver fibrosis by transient elastography. The score includes age, gender and six standard laboratory variables. The overall AUC of the score for predicting 10-year liver-related mortality was 0-90 (0-88-0-91) compared to 0.84 (0-82-0-86) for the FIB-4 consisting of liver age and three laboratory values.(Serra-Burriel, Juanola et al. 2023)
Histopathology as the gold-standard of fibrosis staging is still used whenever exact fibrosis staging is needed, particularly in clinical trials.
Table 1. Calculation of non-invasive fibrosis scores| NFS | -1.675 + 0.037 x age (years) + 0.094 x BMI (kg/m2) + 1.13 x IFG/diabetes (yes = 1, no = 0) + 0.99 x AST/ALT ratio – 0.013 x platelet (x109/L) – 0.66 x albumin (g/dL) |
| FIB-4 | Age (years) x AST (U/L) Platelet counts (109/L) x √ALT(U/L) |
Notice
Ultrasound (US) should be used as primary imaging in patients with suspected MASLD can be used, but does not allow a differentiation between simple steatosis and MASH (steatohepatitis).
Magnetic resonance-based methods (MR-PDFF, MR-S) can be performed to quantify fat in the liver, but are not suitable for comprehensive diagnostics due to cost and availability
Computed tomography (CT) should not be used in the primary diagnosis of MASLD.
Non-invasive fibrosis scores such as FIB-4 or the NAFLD Fibrosis Score (NFS) are used for risk assessment for the primary evaluation of high-risk patients in whom fatty liver has been detected or who have elevated liver values (GOT, GPT and/or γGT). Suitable as well are ultrasound-based elastography methods.
A liver biopsy should only be performed if fibrosis needs to be reliably detected or ruled out (e.g. in the context of studies) or to rule out/prove other liver diseases.
FIB-4 = [age (years) × AST (U/L)] / [Platelets (109/L) × (ALT (U/L)1/2]
Therapy
a) Diet, physical exercise and lifestyle recommendations
A decrease in body weight is accompanied by regression of steatosis in overweight or obese MASLD patients.(2016) The decrease in steatosis and ALT/GPT is proportional to weight loss; there is a clear relationship between degree of weight loss and effect.(Parry and Hodson 2020) It is irrelevant how the weight loss is achieved. The evaluation of paired liver biopsies from MASH patients before and after weight reduction shows that a weight reduction of at least 10% must be achieved in order to achieve regression of fibrosis and complete resolution of MASH/NASH.(Vilar-Gomez, Martinez-Perez et al. 2015) Systematic reviews and guidelines also come to this conclusion. Less weight loss primarily leads to an improvement in steatosis and transaminases. In normal-weight MASLD patients, a controlled study showed 50% remission of steatosis if a weight reduction of 3-5% was achieved.
A combination of hypocaloric nutrition and exercise is recommended based on existing evidence. A 16-week lifestyle intervention with hypocaloric nutrition and aerobic exercise resulted in significant reductions in weight and portal hypertension in overweight or obese patients with liver cirrhosis; a weight reduction of at least 10% was associated with a 23% reduction in hepatic venous pressure gradient (HVPG).(Berzigotti, Albillos et al. 2017) No long-term results from studies on lifestyle intervention are available to date on the question of regression of existing MASH cirrhosis or prevention of disease progression including the development of HCC.
Overall, a weight reduction of at least 10% is extremely effective in the treatment of MASH (90% cure rate), but in clinical practice a goal that has only been achieved by 10% of patients. Concepts such as web-based training, text messaging or increased motivation through donations for charitable purposes are new approaches to solving this dilemma.
Exercise should be done to reduce fatty liver and increase the anti-inflammatory effect of weight loss. An improvement in the necro-inflammatory process has not yet been proven. Determinations of liver fat using 1H-MRS show that aerobic exercise without changing body weight resulted in a decrease in hepatic fat content. Meta-analyses show that aerobic training and/or isometric training in MASLD patients even improved transaminases and hepatic fat content independently of weight loss.(Hashida, Kawaguchi et al. 2017) Both training concepts are apparently equally effective. Aerobic or isometric training can reduce hepatic fat content and insulin resistance. It therefore seems plausible to recommend such training to normal-weight MASLD patients in order to improve steatosis and insulin sensitivity. A meta-analysis came to an end, that both forms of training are equally effective with regard to hepatological endpoints, but that isometric training is less stressful for people with poor cardiorespiratory fitness.(Hashida, Kawaguchi et al. 2017)
According to the WHO exercise guidelines of November 25th, 2020, published at https://www.who.int/publications/i/item/9789240015128, patients with MASLD and a BMI >20 and <25kg/m2 should exercise at least 150 to 300 minutes per week engage in moderate-intensity aerobic physical activity or at least 75 to 150 minutes of vigorous-intensity aerobic physical activity. Alternatively, an equivalent combination of moderate- and vigorous-intensity activity during the week can also be considered.
The rationale for weight reduction is an improvement in comorbidity risks, in transaminases and in liver histology (necroinflammation). Mediterranean diet (ME) can improve steatosis and insulin sensitivity. The results of seven interventional and four observational studies suggest that ME has beneficial effects on body weight and insulin sensitivity and hepatic steatosis.(Roeb, Canbay et al. 2022) However, the available data on the preventive effectiveness of ME with regard to the occurrence of MASLD is less clear. Data from the Framingham study show a reduced risk of developing MASLD in people with high adherence to ME; here, a high-quality diet such as ME was effective, especially in the presence of genetic risk factors.(Suárez, Boqué et al. 2017)
Overweight or obese MASLD patients should be advised on a hypocaloric diet in accordance with the guidelines for the treatment of obesity (AWMF Guideline Adiposity Prevention and Therapy 050-001).(Garvey, Mechanick et al. 2016) The caloric target is 1200 kcal/d for women and 1400-1500 kcal/d for men, corresponding to a reduction of -500 to -1000 kcal/d. The combination of hypocaloric nutrition with aerobic or isometric training acts synergistically and increases the effectiveness in improving steatosis and necroinflammatory activity.(Vilar-Gomez, Martinez-Perez et al. 2015) When energy balance was altered to the same extent by either a hypocaloric diet alone or a combination of less restrictive nutrition and exercise, participants in a systematic study each achieved the same weight loss (-10%) and the same improvement in transaminases, liver fat, and insulin sensitivity. Both interventions are also effective on their own if the other variable - weight or physical activity - is held constant.
The study situation shows no advantage for a specific composition of the macro nutrients fat or carbohydrates of a hypocaloric diet with regard to weight reduction or improvement of transaminases or histological changes in MASLD. This also applies to the use of formula diets, so-called very low energy diets (VLED), as a meal replacement.(Deibert, Lazaro et al. 2019) Using a VLED (800 kcal/d), more than 80% of a Munich cohort achieved a weight loss of at least 10% in 52 weeks, accompanied by significant improvements in transaminases, Fatty Liver Index and NAFLD Fibrosis Score.(Hohenester, Christiansen et al. 2018) A high-protein diet may be beneficial. In obese patients with T2DM, an isocaloric high-protein diet led to an improvement in steatosis, insulin sensitivity, and BMI after 6 weeks.(Markova, Pivovarova et al. 2017) The rapidly increasing obesity prevalence in recent decades has been associated with the increasing consumption of fructose and fructose-containing corn syrup in processed foods and beverages.(Stricker, Rudloff et al. 2021) However, meta-analyses did not show that fructose consumption as part of a normocaloric diet promotes the development or progression of MASLD. In a double-blind study in obese subjects, excess caloric intake, but not fructose versus isocaloric amounts of glucose, was associated with increases in hepatic fat content and transaminases.(Johnston, Stephenson et al. 2013)
Compared to metabolically healthy people, also people of normal weight who are metabolically ill have a more than three-fold increased risk of mortality or cardiovascular events. A controlled study of normal-weight (BMI 22.7 kg/m2) MASLD patients in Hong Kong showed that a hypocaloric diet with a weight reduction of 3-5% led to remission of MASLD in 50% (measured by determining hepatic fat content using 1H-MRS).(Wong, Wong et al. 2018)
b) Alcohol and coffee, stimulants
Retrospective studies that show a beneficial effect of moderate alcohol consumption on health must be evaluated critically, since they only examined associations and not causalities. In addition, prospective data from animal experiments clearly showed a negative influence of alcohol on, for example, diet-induced fatty liver. This observation could also be made in MASLD patients who showed accelerated fibrosis progression due to alcohol consumption.(Ajmera, Terrault et al. 2017) Finally, a retrospective study showed that patients with MASH cirrhosis who consume alcohol, even in small amounts, had a significantly higher risk of developing HCC.(Ascha, Hanouneh et al. 2010) Alcohol consumption is a significant risk factor for the development of liver cirrhosis, and social alcohol consumption should be avoided completely, especially in advanced stages of the disease. Absolute abstinence is recommended here. Due to the relevant and significant damaging mechanisms of alcohol in metabolic dysfunction associated fatty liver disease, the new nomenclature provides for a separate group for these patients, the metALD.(Rinella, Lazarus et al. 2023)
Systematic reviews and meta-analyses suggest that drinking coffee reduces disease progression and HCC risk. Higher doses of coffee resulted in a higher risk reduction. The protective agents from coffee and the molecular mechanisms of HCC prevention have so far remained unclear. Positive effects related to coffee consumption can be derived from epidemiological studies.(Poole, Kennedy et al. 2017) A protective effect of coffee consumption was shown here in relation to the risk of suffering from MASLD and also in relation to the fibrosis stage, although there are no controlled studies on the subject. In a pooled meta-analysis with a total of 11 studies, people who drank coffee had a relative risk of 0.77 (95% CI 0.60-0.98) of suffering from MASLD. In addition, there was a significantly reduced risk of advanced liver fibrosis compared to patients who did not drink coffee (RR 0.68).(Hayat, Siddiqui et al. 2021)
Figure 2 summarises the diet, physical exercise and lifestyle recommendations in case of MASLD diagnosis.
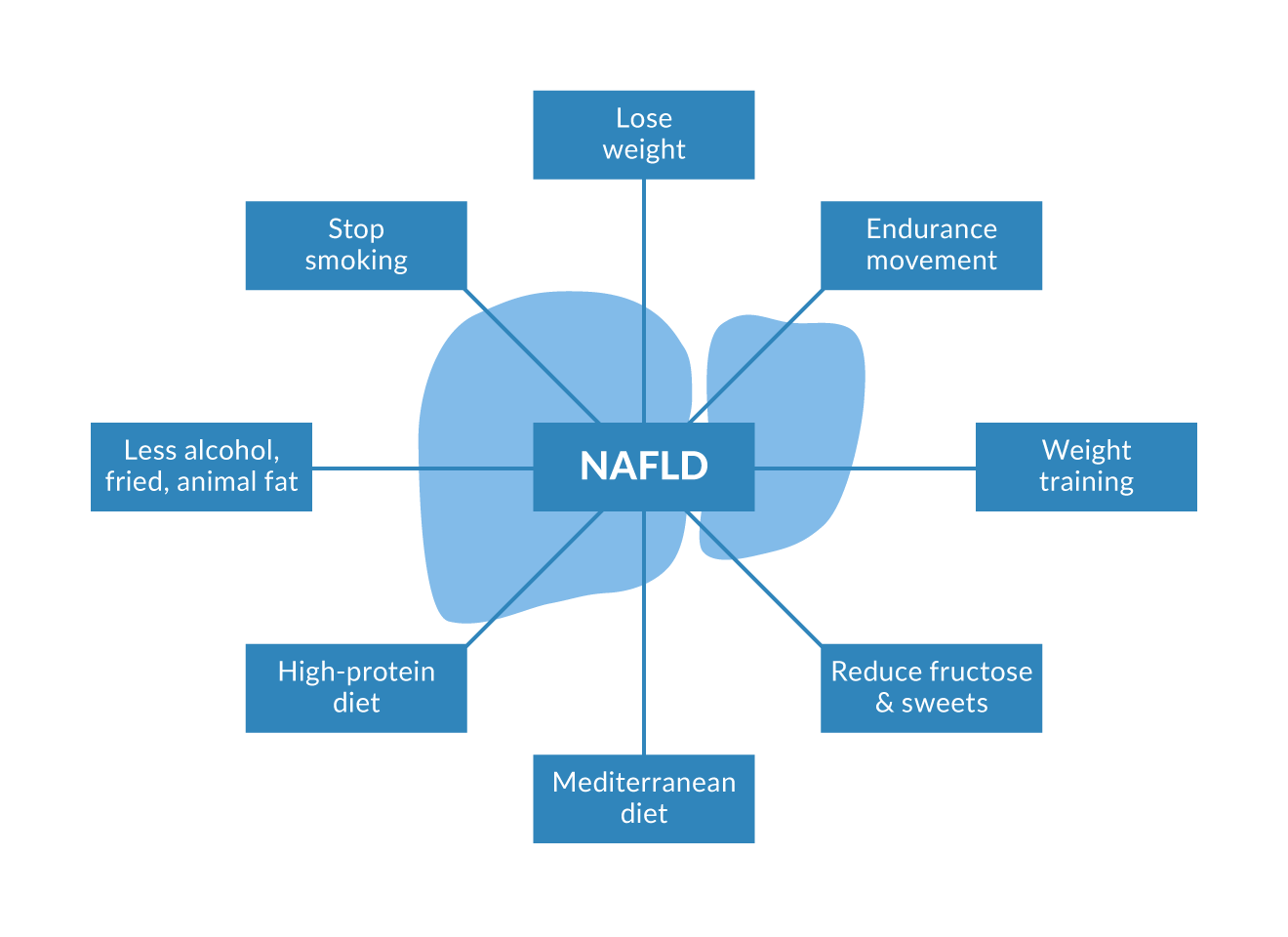 Figure 2. Recommendations for life style changes, cornerstone of medical recommendations. All recommendations are discussed intensively within the text.
Figure 2. Recommendations for life style changes, cornerstone of medical recommendations. All recommendations are discussed intensively within the text.
c) Pharmacological treatment
Specific MASH treatment
The general use of drugs such as ursodeoxycholic acid, pioglitazone, metformin, silymarin or pentoxifylline as well as dietary supplements such as vitamin E or omega-3 fatty acids should not take place based on the current data for the treatment of MASLD.(Roeb, Canbay et al. 2022) The basic therapy of MASLD is based on the underlying comorbidities where several drugs are approved with additional benefit an MASLD. Figure 3 gives an overview of these available drug therapies based on the fibrosis stage according to (Roeb, Canbay et al. 2022)
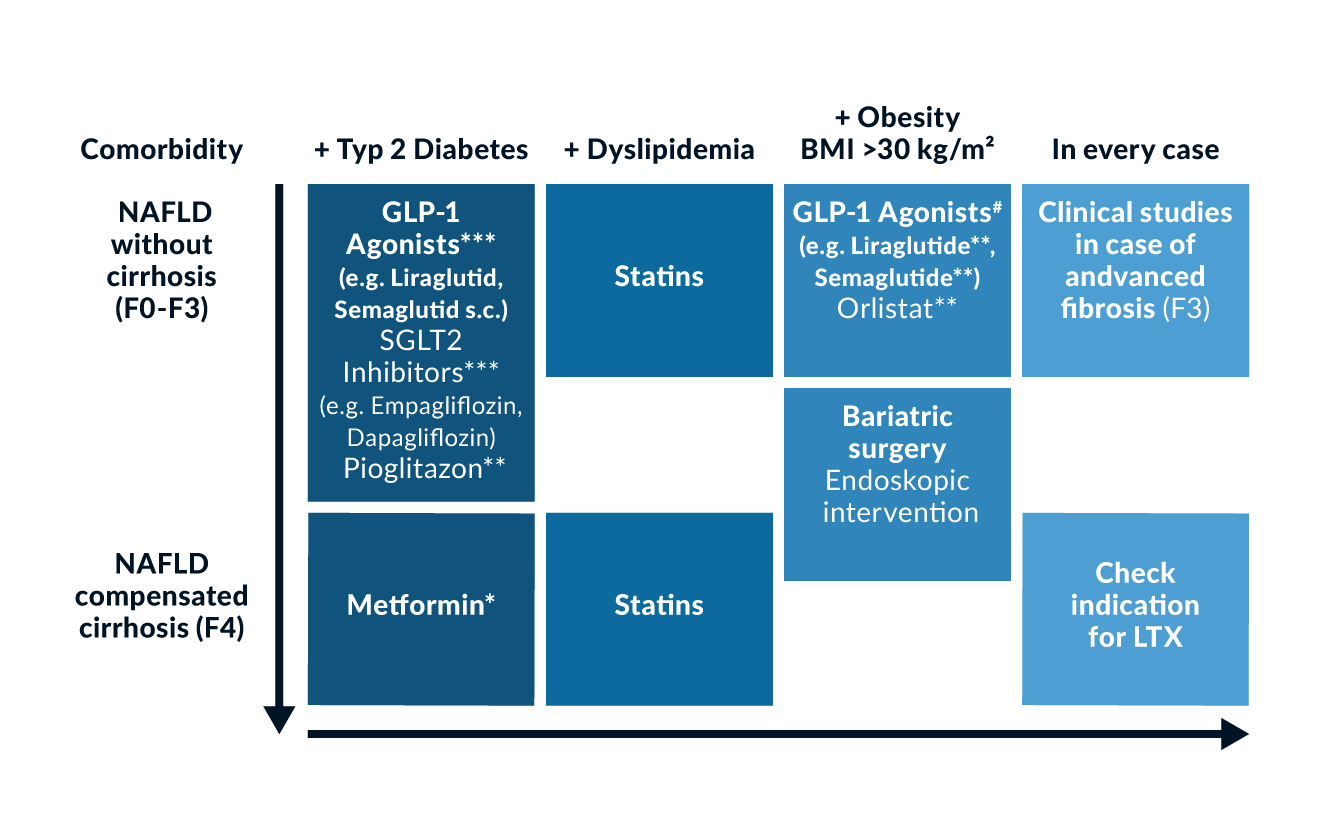 Figure 3. Recommendations to treat MASLD with regard of the fibrosis stage and accompanying comorbidities. *in case of GFR >30ml/min; **currently not reimbursable in statutory health insurance; ***approved in combination with metformin; #approval for liraglutide and semaglutide so far. Modified with regard to (Roeb, Canbay et al. 2022).
Figure 3. Recommendations to treat MASLD with regard of the fibrosis stage and accompanying comorbidities. *in case of GFR >30ml/min; **currently not reimbursable in statutory health insurance; ***approved in combination with metformin; #approval for liraglutide and semaglutide so far. Modified with regard to (Roeb, Canbay et al. 2022).
MASLD patients with comorbidities, such as type 2 diabetes mellitus or obesity, should be treated with lifestyle modifications and, if indicated, with specific medications.(Roeb, Canbay et al. 2022) For the substance class of incretins/incretin mimetics and SGLT2 (sodium-glucose cotransporter 2) inhibitors, favourable effects on MASLD have been demonstrated (in addition to their effect in the approved indication).(Roeb, Canbay et al. 2022, Newsome and Ambery 2023, Loomba, Hartman et al. 2024, Roeb, Canbay et al. 2024, Lin, Huang et al. 2025, Sanyal, Newsome et al. 2025) In MASLD patients with comorbidities, the use of GLP-1 receptor agonists (GLP-1-RA), SGLT2 inhibitors, or dual incretin mimetics as standard therapy in clinical care should therefore be considered and is possible as an adjunctive treatment. These substances also have positive effects on the improvement of MASH and fibrosis.(Loomba, Hartman et al. 2024, Zachou, Flevari et al. 2024, Lin, Huang et al. 2025, Sanyal, Newsome et al. 2025).
An increased risk of disease progression has been described for MASLD patients with type 2 diabetes, obesity, at least one additional cardiometabolic risk factor, and in patients with persistently elevated transaminases.(Dulai, Singh et al. 2017, Roeb, Canbay et al. 2022, Chalasani, Vilar-Gomez et al. 2024, EASL-EASD-EASO 2024, Xiao, Liu et al. 2024, Kim, Cho et al. 2025, Younossi, Zelber-Sagi et al. 2025) In general, the number of cardiometabolic risk factors sums up on the risk to develop liver-related events. (Park, Cheuk-Fung Yip et al. 2025) In these patient groups, further investigation regarding significant fibrosis and treatment indication for resmetirom is indicated.(Roeb, Canbay et al. 2022, EASL-EASD-EASO 2024)
Resmetirom was approved by the FDA in 2024 and by the EMA in June 2025 for the treatment of adult patients with MASH and moderate to advanced fibrosis (F2/F3) in combination with diet and exercise.(https://www.dgvs.de/wp-content/uploads/2023/04/ Amendment-Resmetirom-Leitlinie-S2k-MASLD_14.11.2025.pdf) The recommended dosage of resmetirom is 100 mg/day for individuals weighing 100 kg or more and 80 mg/day for individuals weighing less than 100 kg.
In a Phase 3 study of adults diagnosed with MASH and predominantly F2/F3 fibrosis (F1B fibrosis 5-6%), treatment with resmetirom (80 mg or 100 mg per day) resulted in significantly more frequent resolution of MASH without worsening of fibrosis (25.9% and 29.9% versus 9.7%) and fibrosis improvement without worsening of MASH (24.2% and 25.9% versus 14.2%) at week 52 (p <0.0001 for both endpoints).(Harrison, Bedossa et al. 2024) Treatment with resmetirom had no significant effect on body weight, glucose levels, or insulin resistance. However, resmetirom led to a significant reduction in atherogenic lipids in plasma, such as LDL cholesterol, triglycerides, apolipoprotein B, and lipoprotein(a).(Harrison, Bedossa et al. 2024)
Semaglutide received an accelerated FDA approval in August 2025 for the treatment of MASH with moderate to advanced fibrosis (corresponding to stages F2-F3 of fibrosis), based on preliminary results from the Phase 3 ESSENCE study, in which 72 weeks of subcutaneous injection of 2.4 mg/week resulted in the achievement of both primary histological endpoints: 1) resolution of MASH without worsening fibrosis (62.9% vs. 34.3% on placebo, p < 0.001) and 2) ≥1-stage reduction in liver fibrosis without worsening MASH (36.8% vs. 22.4% on placebo, p < 0.001). Semaglutide demonstrated a favourable hepatic safety profile in the ESSENCE study, with no discontinuations due to elevated liver enzymes. The most common adverse events were gastrointestinal complaints (nausea, diarrhoea, constipation, vomiting), which were generally mild and transient.
For both drugs, current approval is conditional and final approval is still pending due to a lack of long-term results. In the attempt to avoid invasive diagnosis of histologically proven F2 or F3 MASH, non-invasive test (NIT) criteria have been defined for at-risk MASH with target ranges and grey zones necessitating further clinical evaluation, particularly to exclude cirrhosis: VCTE (10-15 with grey zones 8-10 and 15-19.9 kPa), MRE (3.1-4.4 kPa; grey zone up to 4.9 kPa) or ELF (9.2-10.5, grey zone up to 11.3) (Noureddin M et al. Clin Gastroenterol Hepatol 2024; Jul 20:S1542-3565(24)00667-0; Kim VL et al. Hepatology 2025; 81:312–320; https://www.dgvs.de/wp-content/uploads/2023/04/ Amendment-Resmetirom-Leitlinie-S2k-MASLD_14.11.2025.pdf). The combination of semaglutide at a dose of 2.4 mg/week with resmetirom has not been studied so far.
Basic therapy of concomitant disease
Due to the beneficial effects on MASH, non-cirrhotic MASLD patients with type 2 diabetes should be given (metformin plus) glucagon-like peptide 1 (GLP-1) agonists, e.g. semaglutide can be used. The use of sodium glucose dependent transporter 2 (SGLT2) inhibitors, e.g. drugs such as empagliflozin and dapagliflozin or the thiazolidinedione pioglitazone may be considered in these patients. Patients with MASH-associated cirrhosis and type 2 diabetes with compensated Child A cirrhosis and normal renal function may receive metformin.
GLP-1 agonists and SGLT2 inhibitors are only approved in combination with metformin (or as monotherapy in the case of metformin intolerance). The German guideline for type 2 diabetes from 2020 provides for a combination therapy of metformin + SGLT2 inhibitors or GLP-1 agonists for type 2 diabetes with risk factors; without risk factors even metformin monotherapy:
https://www.leitlinien.de/mdb/downloads/nvl/diabetes-mellitus/dm-2aufl-konsultation.pdf. In the current European recommendations, metformin is only listed as the drug of first choice for T2DM therapy if there are no cardiovascular complications.(Cosentino, Grant et al. 2020) GLP-1 analogues showed positive effects in cardiovascular endpoint studies and have comparatively few contraindications. Therapy with sodium glucose dependent transporter 2 (SGLT2) inhibitors showed a significant improvement in liver fat content in patients with MASLD and T2D in proof-of-concept (phase 2a) studies. Data from randomised controlled studies on the effect of SGLT2 inhibitors on liver histology (phase 2b) are currently not available. SGLT2 inhibitors also show positive effects in cardiovascular and renal endpoint studies. The side effects mainly concern urogenital infection, dehydration and the masking of the symptoms and findings of diabetic ketoacidosis.
Furthermore, there are a number of older studies on the use of pioglitazone in patients with MASH who have either impaired glucose tolerance or T2DM. In an 18-month placebo-controlled study therapy with pioglitazone showed greater reductions in liver fat content, more frequent resolution of MASH/NASH and also a greater improvement in fibrosis.(Cusi, Orsak et al. 2016) However, pioglitazone is contraindicated, particularly in heart failure (NYHA I-IV) and bladder carcinoma. Caution is also advised in those with an increased risk of bone fractures and higher degrees of obesity, since pioglitazone promotes weight gain. These safety concerns explain the overall lower strength of recommendation for pioglitazone.
There is currently insufficient experience with the possible use of GLP1 agonists, SGLT2 inhibitors or pioglitazone in patients with MASH-associated liver cirrhosis. SGLT2 inhibitors should be reduced in dose at GFR <60 mL/min and discontinued at <45 mL/min.
Other antidiabetics such as metformin, dipeptidyl peptidase IV inhibitors or insulin have so far not shown any specific advantages with regard to the therapy of MASLD. However, large retrospective studies have reported that there is a reduced risk of developing HCC in MASLD patients taking metformin. Also, in patients with MASH-associated compensated liver cirrhosis Child A, taking metformin for diabetic treatment is associated with a reduced risk of hepatic decompensation and HCC. Thus, metformin can be used as the basis of T2D treatment even in compensated liver cirrhosis (up to a dose of 2 g/d with normal renal function).(Vilar-Gomez, Calzadilla-Bertot et al. 2021) Metformin is contraindicated at GFR below 30 mL/min. However, there are no prospective controlled studies on the use of metformin in liver cirrhosis.
A placebo-controlled study of patients with MASH and T2DM showed that vitamin E (800 IU/day) resulted in a greater reduction in liver fat content and more frequent MASH/NASH reduction without improvement in fibrosis.(Bril, Biernacki et al. 2019) The risk of increased mortality and morbidity with supplementation with vitamin E (see above) limits its use, particularly in patients with diabetes mellitus.
If a lipid metabolism disorder is present in MASLD patients, this should be treated effectively. In view of the overall beneficial effects, statins can also be used in MASLD patients with compensated liver cirrhosis.
The drug orlistat, which is approved for the treatment of obesity, also showed positive effects on the course of MASH. Such data (beneficial influence on MASLD) are not available for other approved weight reduction medicinal products.
d) Novel pharmacological approaches
Given the epidemic increase, regulatory agencies have defined an unmet medical need and implemented initiatives to expedite the development of drugs for MASH treatment (US Food and Drug Administration. Noncirrhotic Non-Alcoholic Steatohepatitis with Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry. https://wwwfdagov/media/119044/download. December 2018).
Taking the prognostic value of the fibrosis stage into account, the FDA encourages sponsors to focus future drug development on the area of greatest need and potential effect on health, which is the stage of non-cirrhotic MASH with liver fibrosis (US Food and Drug Administration. Noncirrhotic Non-Alcoholic Steatohepatitis with Liver Fibrosis: Developing Drugs for Treatment Guidance for Industry. https://wwwfdagov/media/119044/download. December 2018).
New drugs for MASH treatment in clinical phase 2 and 3 development act on different pathophysiological processes such as metabolism/steatosis, inflammation or fibrosis. However, monotherapy with these drugs led to a histological resolution of MASH/NASH at the maximum of 32% of patients compared to a 10-15% response in placebo.(Dufour, Caussy et al. 2020) Therefore, the future in MASH therapy will putatively be a combination therapy of two different drug classes with complementary effects. Current and expected drug classes for MASH treatment include agonists of nuclear receptors such as FXR agonists (including FGF19), PPAR agonists, thyroid hormone receptor-ß agonists as well as analogues of enterohepatic hormones such as incretins and FGF21(Loomba, Sanyal et al. 2023) or SGLT2 inhibitors.(Rau and Geier 2021) Therapeutic targets for MASH are summarised in figure 4.
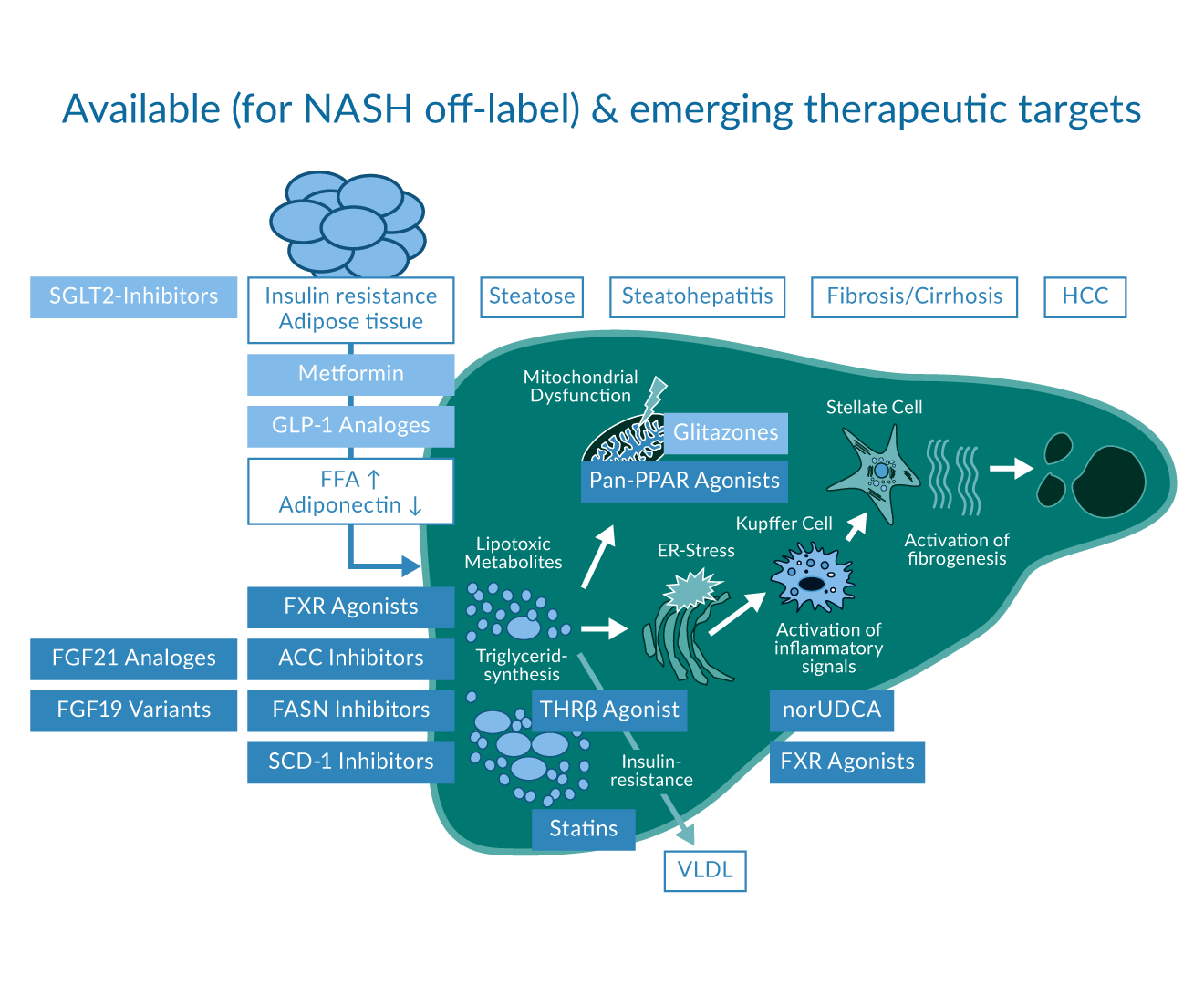 Figure 4. Emerging therapeutic targets and substances which are already available (off label use in case of MASLD) adapted from [85].
Figure 4. Emerging therapeutic targets and substances which are already available (off label use in case of MASLD) adapted from [85].
Obeticholic acid (OCA) as agonist of the bile acid receptor farnesoid X receptor (FXR) has investigated in two phase 3 studies in MASH patients. Although interim analysis showed an improvement in fibrosis in both treatment groups compared to placebo (Younossi, Ratziu et al. 2019, Sanyal, Ratziu et al. 2023) the drug did not achieve conditional approval by the FDA due to its risk-to-benefit ratio (including LDL increase and pruritus) and is currently not developed further.
Tropifexor is another potent oral FXR agonist, which is structurally not related to bile acids. Anti-inflammatory and anti-steatotic effects were observed in an adaptive phase 2a/b trial (FLIGHT-FXR). Decreases from baseline in ALT and hepatic fat fraction measured by MRI-PDFF were sustained from week 12 up to week 48.(Sanyal, Lopez et al. 2023, Fiorucci, Urbani et al. 2025) Interestingly, pruritus was also observed for compound despite its non-steroidal nature.
Cilofexor is another non-steroidal FXR agonist with dose-dependent, anti-steatotic effect in MRI-PDFF in a phase 2 study. Pruritus was also observed in the high dose treatment arm.(Patel, Harrison et al. 2020)
Nor-Ursodeoxycholic acid (norUDCA), a synthetic bile acid characterised by anti-inflammatory properties without relevant FXR agonistic effects, is currently investigated in a phase 2b study (EudraCT 2018-003443-31).
Aldafermin (NGM282) is a nontumourigenic variant of the FXR target gene fibroblast growth factor FGF19 inhibits de novo lipogenesis, improves insulin sensitivity, corrects mitochondrial dysfunction, and reduces hepatic inflammation and fibrosis. In a phase 2a study over 12 weeks Aldafermin treatment led more often to a 5% or more reduction of hepatic fat fraction (MRI-PDFF) compared to placebo (74% for NGM282 3mg, 79% for NGM282 6mg and 7% for placebo).(Harrison, Neff et al. 2021) However, in phase 2b, no significant dose response on improvement in liver fibrosis with no worsening of MASH/NASH has been detected (19% in the placebo group, 31% 0.3 mg aldafermin, 15% 1.0 mg and 30% 3.0 mg; p=0.12).(Harrison, Abdelmalek et al. 2022) The most common adverse events were gastrointestinal (e.g. diarrhoea, nausea, frequent bowel movements and abdominal pain).
Efruxifermin (EFX) is a long-acting fibroblast growth factor FGF21 analogue based on a human IgG1 Fc-fusion protein with beneficial effect on glucose- and lipid metabolism. It mimics the balanced potency of endogenous FGF21 over the three FGF receptors 1c, 2c and 3c. In the phase 2a BALANCED study all Efruxifermin dose groups (28mg, 50mg, 70mg) met the primary endpoint with absolute changes in hepatic fat fraction of −12.3% from baseline compared to 0.3% in the placebo group.(Harrison, Ruane et al. 2021) Consistent with reduced hepatic fat fraction, 78% of Efruxifermin-treated patients in this study were NAS responders (≥2 points without worsening of fibrosis) and 48% had MASH/NASH resolution without worsening of fibrosis. Beneficial effects on cholesterol and glucose metabolism have been confirmed. In the recently published phase 2b HARMONY study, both 50mg and 28mg doses of Efruxifermin led to at least a one stage improvement in liver fibrosis with no worsening of MASH/NASH by week 24 (41% and 39%, respectively) compared to 20% for placebo.(Noureddin, Frias et al. 2025) Based on these data, Efruxifermin has received a Breakthrough Therapy Designation from the FDA.
As outlined above, Glucagon-like peptide-1 (GLP-1) analogues are established drugs in diabetes and obesity therapy with beneficial metabolic effects particularly on glucose metabolism. Semaglutide, a new generation GLP-1 agonist, has already been approved by the FDA for MASH with fibrosis grades 2 and 3.(Bansal, Patton et al. 2025)
Resmetirom is a highly selective thyroid hormone receptor ß (THRß) agonist was approved by the FDA and, in June 2025, also by the EMA for MASH with fibrosis grades 2 and 3. (https://www.dgvs.de/wp-content/uploads/2023/04/ Amendment-Resmetirom-Leitlinie-S2k-MASLD_14.11.2025.pdf) THRß agonists target dyslipidaemia but have also been shown to reduce hepatic steatosis, improving insulin sensitivity, promoting liver regeneration and reducing apoptosis in preclinical studies.
In the phase 2 MAESTRO trial, 955 MASH F2-F3 patients were analysed in the interim analysis that showed a significant effect of both doses of Resmetirom 80mg and 100mg on fibrosis improvement by at least one stage without worsening of MASH/NASH (24% and 26%, respectively) as compared to placebo (14%; p<0.0001) after 52 weeks of treatment.(Harrison, Taub et al. 2023) Similarly, a significant improvement in MASH/NASH without worsening of fibrosis has been detected (26% and 30% for Resmetirom 80/100mg compared to 10% in placebo; p<0.0001). A decrease in LDL cholesterol could be observed as secondary endpoint. Resmetirom was generally well tolerated with mild, transient diarrhoea and nausea as the most common gastrointestinal adverse events. Results of the recently published phase 3 study show that resmetirom was safe and well tolerated in adults with NASH, which favours further clinical development.(Harrison, Taub et al. 2023)
VK2809 is another THRß agonist in clinical development (Phase 2b VOYAGE study ongoing).
PPAR (peroxisome proliferator-activated receptor) agonists exert beneficial effects in glucose as well as lipid metabolism and are therefore an interesting drug class for NASH treatment. As outlined above for Pioglitazone, PPAR agonists have traditionally been used for the treatment of patients with metabolic syndrome to lower serum triglyceride and glucose levels. Lanifibranor (IVA337) is a pan-PPAR agonist with activation of three different receptor isoforms α, δ and γ. In a phase 2b NATIVE study involving 247 NASH patients with F1-F3 fibrosis, Lanifibranor has met the primary endpoint with a reduction of the steatosis activity fibrosis score (SAF) by at least 2 points in SAF-A with no worsening of fibrosis (1200mg versus placebo, 55% vs. 33%, P=0.007; 800mg versus placebo, 48% vs. 33%, P=0.07).(Francque, Bedossa et al. 2021) As a key secondary endpoint, improvement in fibrosis of at least one stage without worsening of MASH/NASH also favoured both Lanifibranor doses over placebo (48% and 34%, respectively, vs. 29%). As expected for this pan-PPAR agonist, lipid, inflammatory and fibrosis biomarkers were improved in the Lanifibranor groups. Lanifibranor showed an overall favourable tolerability profile. However, it is worth to note that weight gain occurred more frequently with Lanifibranor than with placebo. These findings support further assessment of Lanifibranor in the ongoing phase 3 trial (NATIV3). Elafibranor (GFT505), a dual PPAR α/δ agonist, did not demonstrate a statistically significant effect on the primary endpoint of MASH/NASH resolution without worsening of fibrosis in the respective phase 3 study (RESOLVE-IT), which has been terminated early.
Aramchol is a liver-targeted steroyl-CoA desaturase (SCD-1) inhibitor. SCD-1 represents a key enzyme in hepatic lipogenesis that converts saturated fatty acids into monounsaturated fatty acids. In a 52-week phase 2b study (ARREST), Aramchol showed liver fat reduction, biochemical improvement, MASH/NASH resolution and fibrosis reduction in a dose dependent manner.(Ratziu, de Guevara et al. 2021) Aramchol is currently being evaluated in an ongoing phase 3 programme ARMOR clinical trial (open-label phase followed by a randomised, double-blinded and placebo-controlled phase). As announced in a recent press release, histological improvement in fibrosis by at least one stage was demonstrated in 39% of subjects in the open-label part (Galmed Pharmaceuticals, Press Release January 4 2023).
TVB-2640 represents a novel, first-in-class, fatty acid synthase (FASN) inhibitor which has recently been tested in a phase 2 study in MASH patients over 12 weeks.(Loomba, Mohseni et al. 2021) Following the same approach as Aramchol to target a key enzyme hepatic lipogenesis, a dose-dependent reduction of liver fat fraction (-9.6% and -28.1% at 25mg and 50mg respectively) has been observed with MRI-PDFF together with favourable metabolic effects in serum.
Given the complex pathogenesis of MASH, it is intuitive that multiple mechanistic pathways could be targeted to achieve an optimal treatment response. Several treatment combinations are currently tested in MASH. Most combination therapies include an FXR agonist as therapeutic backbone. In a phase 2 study (ATLAS trial) the safety and efficacy of monotherapy and dual combination regimens of Cilofexor 30 mg, Firsocostat 20 mg and Selonsertib 18 mg in patients with advanced MASH fibrosis and cirrhosis (F3-F4) were evaluated. The monotherapy arm with Selonsertib was discontinued shortly after the negative results of Selonsertib monotherapy. In the ATLAS trial a ≥1-stage improvement in fibrosis without worsening of MASH/NASH after 48 weeks of treatment was numerically higher in the combination therapy group (Cilofexor and Firsocostat) compared with placebo (21% vs 11%, p=0.17), respectively. The primary endpoint was not met probably due to a small sample size.(Loomba, Noureddin et al. 2021) Subsequently, the two active compounds from this trial (Cilofexor 30-100mg, Firsocostat 20mg) have been combined with Semaglutide 0.24-2.4mg in a phase 2 study in F2-3 MASH patients over 24 weeks.(Alkhouri, Herring et al. 2022) Despite similar reductions in body weight like in Semaglutide alone, combination regimens resulted in greater improvements in hepatic steatosis as assessed by MRI-PDFF (−9.8 to −11.0% vs. −8.0%).(Loomba, Noureddin et al. 2021) Side effects in combination were similar to the single compounds. Other trials are currently testing the combination of Tropifexor with the SGLT1/2 Inhibitor Licogliflozin or with the Leukotriene A4 hydrolase inhibitor LYS006.
e) Modification of the intestinal microbiome
In a randomised study, the supplementation of a combination of pro- and prebiotics showed a change in the microbiome, but no effect on liver fat content or liver stiffness as a surrogate for liver fibrosis.(Scorletti, Afolabi et al. 2020) In lean MASLD patients (n=50), synbiotics showed a benefit in terms of improving non-invasive surrogates of fatty liver and fibrosis over 28 weeks.(Mofidi, Poustchi et al. 2017) Data on the transfer of microbiota are not available.
A recent work investigated the role of the gut-liver axis in MASLD hepatocarcinogenesis. The study suggests that the gut microbiota in MASLD -HCC is characterised by a distinct microbiome or metabolomic profile and can modulate the peripheral immune response.(Behary, Amorim et al. 2021)
f) Bariatric Surgery
An increasing number of studies are investigating endoscopic and laparoscopic methods of bariatric surgery for the treatment of MASLD /MASH. Bariatric surgery improves the serological, imaging, and histological markers of MASLD.(Schmid, Arians et al. 2022, Verrastro, Panunzi et al. 2023) In obesity and MASLD, sleeve gastrectomy, Roux-Y gastric bypass and single-anastomosis gastric bypass can be recommended or performed as metabolic surgical procedures. The adjustable gastric band should not be used in obesity and MASLD due to inferior efficacy.(Roeb, Canbay et al. 2022) Because of the risk of progressive liver failure, the severity of MASLD should be critically considered when an indication for malabsorptive procedures (e.g. biliopancreatic diversion, distal gastric bypass and single anastomosis bypass with a biliopancreatic loop longer than 200 cm) is made. If liver cirrhosis is present, sleeve gastrectomy should preferably be used. Endoscopic procedures can also be used in MASLD and obesity if conservative therapy fails and if bariatric surgery is rejected or contraindicated. Here, the endoscopic intragastric balloon application or the endoscopic gastric sleeve (ESG) may still come into question.(Roeb, Canbay et al. 2022) At the end, it is important to point out that surgical methods represent an ultimate approach after failure of conservative treatment strategy and is reserved to patients with morbid obesity (BMI >40 kg/m2 or BMI >35kg/m2 with serious concomitant disease).
g) Liver transplantation for MASH
Liver transplantation is the final option for patients with end-stage liver disease due to NASH cirrhosis with decompensation or complications of portal hypertension. Due to the increase in the prevalence of MASH, this disease entity is the second most frequent reason for liver transplantation listing in the North-American UNOS network.(Younossi, Stepanova et al. 2021) In the US, it is currently the second most common LTX indication, with an increase of 167% over the period 2003-2014; in Germany the trend is constantly increasing.(Rademacher, Aehling et al. 2021) However, MASH as underlying disease for liver transplantation is also increasing but not exceeding 10% of cases in the European ELTR registry so far.(Haldar, Kern et al. 2019) MASH can recur after liver transplantation, since this does not cure the metabolic defect that causes MASH. Postoperative outcomes may even worsen in the future with an increasing proportion of steatotic (and therefore marginal) donor organs. Furthermore, it can be emphasised that preexisting MASH-related comorbidity like nephropathy and cardiovascular disease in organ recipients with MASH cirrhosis may further limit the long-term outcomes after transplantation.(Canbay, Sowa et al. 2016) Unfavourable metabolic effects of calcineurin inhibitors further impact on this dilemma.
Follow-up of MASLD and MASH patients
Patients with MASH should undergo clinical follow-up and ultrasound surveillance according to their individual risk.(Roeb, Canbay et al. 2022) The intervals of clinical follow-up are based on the progression risk and are therefore stratified by presence of MASH, fibrosis stage and comorbidities. While patients with bland steatosis can be followed on a more liberal basis in 2 to 3 years intervals, those with advanced fibrosis, particularly cirrhosis, should undergo follow-up with tumour surveillance every 6 months. (bouzas 2016, Roeb, Canbay et al. 2022) Although hepatocellular carcinoma may occur even in MASH patients without cirrhosis, the relative incidence of such an event does probably not justify to screen non-cirrhotic MASH patients for HCC on a regular basis.(Kanwal, Kramer et al. 2018, Roeb, Canbay et al. 2022) The HCC incidence in MASH patients without liver cirrhosis is given as 0.02% per year and increases to 1.5% per year in the presence of liver cirrhosis.(Kanwal, Kramer et al. 2018) An elevated FIB-4 score predicts liver cancer development and may be used for risk stratification in MASH patients.(Loosen, Kostev et al. 2022) Follow-up examinations using non-invasive laboratory based tests or elastography allow to monitor for fibrosis progression. Patients with cirrhosis should be screened for gastroesophageal varices according to current practice guidelines.(bouzas 2016, Roeb, Canbay et al. 2022) Current evidence does not support to routinely repeat liver biopsy in patients with MASLD or MASH.(Chalasani, Younossi et al. 2018)
Structured monitoring during resmetirom therapy is necessary at this stage of conditional approval in order to verify efficacy and ensure safety. The intervals are based on the expected pharmacodynamic effects (early lipid and liver enzyme changes, slower fibrosis changes) and potential side effects. Most side effects occurred within the first few weeks to months, especially gastrointestinal symptoms.(Harrison, Bashir et al. 2021, Harrison, Bedossa et al. 2024) Serious liver-related side effects were rare, but in isolated cases transaminase elevations occurred, so monitoring of liver enzymes (ALT, AST, GGT, bilirubin if necessary) appears to be advisable. Due to the significant reduction in LDL and changes in lipid metabolism under resmetirom, adjustments to statin therapy are necessary. Non-invasive markers (e.g., VCTE) can provide indications of fibrosis regression or progression and guide the indication for further diagnostics (e.g., liver biopsy); due to the slow changes in liver fibrosis under therapy with resmetirom, a check-up after 12 months of therapy appears sufficient, provided there are no clinical indications of deterioration.(Harrison, Bedossa et al. 2024) (https://www.dgvs.de/wp-content/uploads/2023/04/ Amendment-Resmetirom-Leitlinie-S2k-MASLD_14.11.2025.pdf)
References
(2016). "EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease." Diabetologia 59(6): 1121-1140.
(2016). "EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease." J Hepatol 64(6): 1388-1402.
Ajmera, V. H., N. A. Terrault and S. A. Harrison (2017). "Is moderate alcohol use in nonalcoholic fatty liver disease good or bad? A critical review." Hepatology 65(6): 2090-2099.
Alkhouri, N., R. Herring, H. Kabler, Z. Kayali, T. Hassanein, A. Kohli, R. S. Huss, Y. Zhu, A. N. Billin, L. H. Damgaard, K. Buchholtz, M. S. Kjær, C. Balendran, R. P. Myers, R. Loomba and M. Noureddin (2022). "Safety and efficacy of combination therapy with semaglutide, cilofexor and firsocostat in patients with non-alcoholic steatohepatitis: A randomised, open-label phase II trial." J Hepatol 77(3): 607-618.
Alsenbesy, M., M. Rau, J. Weiss, O. Götze and A. Geier (2019). "A 2-step fast-track elastometry service for advanced workup of nonalcoholic fatty liver disease (NAFLD) patients - single-center real-world experience of outpatient clinical practice." Z Gastroenterol 57(10): 1209-1217.
Angulo, P., D. E. Kleiner, S. Dam-Larsen, L. A. Adams, E. S. Bjornsson, P. Charatcharoenwitthaya, P. R. Mills, J. C. Keach, H. D. Lafferty, A. Stahler, S. Haflidadottir and F. Bendtsen (2015). "Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease." Gastroenterology 149(2): 389-397.e310.
Ascha, M. S., I. A. Hanouneh, R. Lopez, T. A. Tamimi, A. F. Feldstein and N. N. Zein (2010). "The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis." Hepatology 51(6): 1972-1978.
Bansal, M. B., H. Patton, T. R. Morgan, R. M. Carr, J. Dranoff and A. M. Allen (2025). "Semaglutide therapy for metabolic dysfunction-associated steatohepatitis: November 2025 updates to AASLD Practice Guidance." Hepatology. 2025 Nov 7. doi: 10.1097/HEP.0000000000001608
Bedogni, G., L. Miglioli, F. Masutti, A. Castiglione, L. S. Crocè, C. Tiribelli and S. Bellentani (2007). "Incidence and natural course of fatty liver in the general population: the Dionysos study." Hepatology 46(5): 1387-1391.
Bedossa, P. (2013). "Current histological classification of NAFLD: strength and limitations." Hepatol Int 7 Suppl 2: 765-770.
Bedossa, P. and F. P. Consortium (2014). "Utility and appropriateness of the fatty liver inhibition of progression (FLIP) algorithm and steatosis, activity, and fibrosis (SAF) score in the evaluation of biopsies of nonalcoholic fatty liver disease." Hepatology 60(2): 565-575.
Bedossa, P., C. Poitou, N. Veyrie, J. L. Bouillot, A. Basdevant, V. Paradis, J. Tordjman and K. Clement (2012). "Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients." Hepatology 56(5): 1751-1759.
Behary, J., N. Amorim, X. T. Jiang, A. Raposo, L. Gong, E. McGovern, R. Ibrahim, F. Chu, C. Stephens, H. Jebeili, V. Fragomeli, Y. C. Koay, M. Jackson, J. O'Sullivan, M. Weltman, G. McCaughan, E. El-Omar and A. Zekry (2021). "Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma." Nat Commun 12(1): 187.
Berzigotti, A., A. Albillos, C. Villanueva, J. Genescá, A. Ardevol, S. Augustín, J. L. Calleja, R. Bañares, J. C. García-Pagán, F. Mesonero and J. Bosch (2017). "Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study." Hepatology 65(4): 1293-1305.
Boursier, J., O. Mueller, M. Barret, M. Machado, L. Fizanne, F. Araujo-Perez, C. D. Guy, P. C. Seed, J. F. Rawls, L. A. David, G. Hunault, F. Oberti, P. Calès and A. M. Diehl (2016). "The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota." Hepatology 63(3): 764-775.
bouzas (2016). "EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease." J Hepatol 64(6): 1388-1402.
Bril, F., D. M. Biernacki, S. Kalavalapalli, R. Lomonaco, S. K. Subbarayan, J. Lai, F. Tio, A. Suman, B. K. Orsak, J. Hecht and K. Cusi (2019). "Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial." Diabetes Care 42(8): 1481-1488.
Canbay, A., J. P. Sowa, W. K. Syn and J. Treckmann (2016). "NASH Cirrhosis - the New Burden in Liver Transplantation: How Should It Be Managed?" Visc Med 32(4): 234-238.
Chalasani, N., E. Vilar-Gomez, R. Loomba, K. P. Yates, A. M. Diehl, B. A. Neuschwander-Tetri, S. Dasarathy, K. V. Kowdley, N. Terrault, L. A. Wilson, J. Tonascia and A. J. Sanyal (2024). "PNPLA3 rs738409, age, diabetes, sex, and advanced fibrosis jointly contribute to the risk of major adverse liver outcomes in metabolic dysfunction-associated steatotic liver disease." Hepatology 80(5): 1212-1226.
Chalasani, N., Z. Younossi, J. E. Lavine, M. Charlton, K. Cusi, M. Rinella, S. A. Harrison, E. M. Brunt and A. J. Sanyal (2018). "The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases." Hepatology 67(1): 328-357.
Chen, V. L., T. R. Morgan, Y. Rotman, H. M. Patton, K. Cusi, F. Kanwal and W. R. Kim (2025). "Resmetirom therapy for metabolic dysfunction-associated steatotic liver disease: October 2024 updates to AASLD Practice Guidance." Hepatology 81(1): 312-320.
Cosentino, F., P. J. Grant, V. Aboyans, C. J. Bailey, A. Ceriello, V. Delgado, M. Federici, G. Filippatos, D. E. Grobbee, T. B. Hansen, H. V. Huikuri, I. Johansson, P. Jüni, M. Lettino, N. Marx, L. G. Mellbin, C. J. Östgren, B. Rocca, M. Roffi, N. Sattar, P. M. Seferović, M. Sousa-Uva, P. Valensi and D. C. Wheeler (2020). "2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD." Eur Heart J 41(2): 255-323.
Cusi, K., B. Orsak, F. Bril, R. Lomonaco, J. Hecht, C. Ortiz-Lopez, F. Tio, J. Hardies, C. Darland, N. Musi, A. Webb and P. Portillo-Sanchez (2016). "Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial." Ann Intern Med 165(5): 305-315.
Deibert, P., A. Lazaro, D. Schaffner, A. Berg, D. Koenig, W. Kreisel, M. W. Baumstark, D. Steinmann, M. Buechert and T. Lange (2019). "Comprehensive lifestyle intervention vs soy protein-based meal regimen in non-alcoholic steatohepatitis." World J Gastroenterol 25(9): 1116-1131.
Dietrich, C. G., M. Rau and A. Geier (2021). "Screening for nonalcoholic fatty liver disease-when, who and how?" World J Gastroenterol 27(35): 5803-5821.
Diogo, D., C. Tian, C. S. Franklin, M. Alanne-Kinnunen, M. March, C. C. A. Spencer, C. Vangjeli, M. E. Weale, H. Mattsson, E. Kilpeläinen, P. M. A. Sleiman, D. F. Reilly, J. McElwee, J. C. Maranville, A. K. Chatterjee, A. Bhandari, K. H. Nguyen, K. Estrada, M. P. Reeve, J. Hutz, N. Bing, S. John, D. G. MacArthur, V. Salomaa, S. Ripatti, H. Hakonarson, M. J. Daly, A. Palotie, D. A. Hinds, P. Donnelly, C. S. Fox, A. G. Day-Williams, R. M. Plenge and H. Runz (2018). "Phenome-wide association studies across large population cohorts support drug target validation." Nat Commun 9(1): 4285.
Dubuquoy, C., A. F. Burnol and M. Moldes (2013). "PNPLA3, a genetic marker of progressive liver disease, still hiding its metabolic function?" Clin Res Hepatol Gastroenterol 37(1): 30-35.
Dufour, J. F., C. Caussy and R. Loomba (2020). "Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges." Gut 69(10): 1877-1884.
Dulai, P. S., S. Singh, J. Patel, M. Soni, L. J. Prokop, Z. Younossi, G. Sebastiani, M. Ekstedt, H. Hagstrom, P. Nasr, P. Stal, V. W. Wong, S. Kechagias, R. Hultcrantz and R. Loomba (2017). "Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis." Hepatology 65(5): 1557-1565.
EASL-EASD-EASO (2024). "EASL-EASD-EASO Clinical Practice Guidelines on the management of metabolic dysfunction-associated steatotic liver disease (MASLD)." J Hepatol 81(3)(Sep): 492-542.
Ekstedt, M., H. Hagström, P. Nasr, M. Fredrikson, P. Stål, S. Kechagias and R. Hultcrantz (2015). "Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up." Hepatology 61(5): 1547-1554.
Eslam, M., A. J. Sanyal, J. George and P. International Consensus (2020). "MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease." Gastroenterology 158(7): 1999-2014 e1991.
Estes, C., Q. M. Anstee, M. T. Arias-Loste, H. Bantel, S. Bellentani, J. Caballeria, M. Colombo, A. Craxi, J. Crespo, C. P. Day, Y. Eguchi, A. Geier, L. A. Kondili, D. C. Kroy, J. V. Lazarus, R. Loomba, M. P. Manns, G. Marchesini, A. Nakajima, F. Negro, S. Petta, V. Ratziu, M. Romero-Gomez, A. Sanyal, J. M. Schattenberg, F. Tacke, J. Tanaka, C. Trautwein, L. Wei, S. Zeuzem and H. Razavi (2018). "Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030." J Hepatol 69(4): 896-904.
Fiorucci, S., G. Urbani, E. Distrutti and M. Biagioli (2025). "Obeticholic Acid and Other Farnesoid-X-Receptor (FXR) Agonists in the Treatment of Liver Disorders." Pharmaceuticals (Basel) 18(9).
Francque, S. M., P. Bedossa, V. Ratziu, Q. M. Anstee, E. Bugianesi, A. J. Sanyal, R. Loomba, S. A. Harrison, R. Balabanska, L. Mateva, N. Lanthier, N. Alkhouri, C. Moreno, J. M. Schattenberg, D. Stefanova-Petrova, L. Vonghia, R. Rouzier, M. Guillaume, A. Hodge, M. Romero-Gómez, P. Huot-Marchand, M. Baudin, M. P. Richard, J. L. Abitbol, P. Broqua, J. L. Junien and M. F. Abdelmalek (2021). "A Randomized, Controlled Trial of the Pan-PPAR Agonist Lanifibranor in NASH." N Engl J Med 385(17): 1547-1558.
Galvan-Martinez, D. H., V. M. Bosquez-Mendoza, Y. Ruiz-Noa, L. D. R. Ibarra-Reynoso, G. Barbosa-Sabanero and M. L. Lazo-de-la-Vega-Monroy (2023). "Nutritional, pharmacological, and environmental programming of NAFLD in early life." Am J Physiol Gastrointest Liver Physiol 324(2): G99-g114.
Garvey, W. T., J. I. Mechanick, E. M. Brett, A. J. Garber, D. L. Hurley, A. M. Jastreboff, K. Nadolsky, R. Pessah-Pollack and R. Plodkowski (2016). "AMERICAN ASSOCIATION OF CLINICAL ENDOCRINOLOGISTS AND AMERICAN COLLEGE OF ENDOCRINOLOGY COMPREHENSIVE CLINICAL PRACTICE GUIDELINES FOR MEDICAL CARE OF PATIENTS WITH OBESITY." Endocr Pract 22 Suppl 3: 1-203.
Geier, A., M. Rau, A. Pathil-Warth, M. von der Ohe, J. Schattenberg, N. Dikopoulos, K. Stein, Y. Serfert, T. Berg, P. Buggisch, M. Demir, E. Roeb, B. Wiebner, H. Wedemeyer, S. Zeuzem and W. P. Hofmann (2023). "Clinical characteristics of patients with non-alcoholic fatty liver disease (NAFLD) in Germany - First data from the German NAFLD-Registry." Z Gastroenterol 61(1): 60-70.
Geier, A., M. E. Rinella, M. M. Balp, S. J. McKenna, C. A. Brass, R. Przybysz, J. Cai, A. Knight, M. Gavaghan, T. Howe, D. Rosen and V. Ratziu (2021). "Real-World Burden of Nonalcoholic Steatohepatitis." Clin Gastroenterol Hepatol 19(5): 1020-1029.e1027.
Geier, A., D. Tiniakos, H. Denk and M. Trauner (2021). "From the origin of NASH to the future of metabolic fatty liver disease." Gut 70(8): 1570-1579.
George, J., R. G. Gish and A. Geier (2021). "MAFLD and Cardiovascular Events: What Does the Evidence Show?" Clin Gastroenterol Hepatol 19(10): 2025-2028.
Hagström, H., J. Vessby, M. Ekstedt and Y. Shang (2023). "99% of patients with NAFLD meet MASLD criteria and natural history is therefore identical." J Hepatol.
Haldar, D., B. Kern, J. Hodson, M. J. Armstrong, R. Adam, G. Berlakovich, J. Fritz, B. Feurstein, W. Popp, V. Karam, P. Muiesan, J. O'Grady, N. Jamieson, S. J. Wigmore, J. Pirenne, S. A. Malek-Hosseini, E. Hidalgo, Y. Tokat, A. Paul, J. Pratschke, M. Bartels, P. Trunecka, U. Settmacher, M. Pinzani, C. Duvoux, P. N. Newsome and S. Schneeberger (2019). "Outcomes of liver transplantation for non-alcoholic steatohepatitis: A European Liver Transplant Registry study." J Hepatol 71(2): 313-322.
Hardy, T., K. Wonders, R. Younes, G. P. Aithal, R. Aller, M. Allison, P. Bedossa, F. Betsou, J. Boursier, M. J. Brosnan, A. Burt, J. Cobbold, H. Cortez-Pinto, C. P. Day, J. F. Dufour, M. Ekstedt, S. Francque, S. Harrison, L. Miele, P. Nasr, G. Papatheodoridis, S. Petta, D. Tiniakos, R. Torstenson, L. Valenti, A. G. Holleboom, H. Yki-Jarvinen, A. Geier, M. Romero-Gomez, V. Ratziu, E. Bugianesi, J. M. Schattenberg and Q. M. Anstee (2020). "The European NAFLD Registry: A real-world longitudinal cohort study of nonalcoholic fatty liver disease." Contemp Clin Trials 98: 106175.
Harrison, S. A., M. F. Abdelmalek, G. Neff, N. Gunn, C. D. Guy, N. Alkhouri, M. R. Bashir, B. Freilich, A. Kohli, A. Khazanchi, M. Y. Sheikh, M. Leibowitz, M. E. Rinella, M. S. Siddiqui, M. Kipnes, S. E. Moussa, Z. H. Younes, M. Bansal, S. J. Baum, B. Borg, P. J. Ruane, P. J. Thuluvath, M. Gottwald, M. Khan, C. Chen, L. Melchor-Khan, W. Chang, A. M. DePaoli, L. Ling and H. D. Lieu (2022). "Aldafermin in patients with non-alcoholic steatohepatitis (ALPINE 2/3): a randomised, double-blind, placebo-controlled, phase 2b trial." Lancet Gastroenterol Hepatol 7(7): 603-616.
Harrison, S. A., M. Bashir, S. E. Moussa, K. McCarty, J. Pablo Frias, R. Taub and N. Alkhouri (2021). "Effects of Resmetirom on Noninvasive Endpoints in a 36-Week Phase 2 Active Treatment Extension Study in Patients With NASH." Hepatol Commun 5(4): 573-588.
Harrison, S. A., P. Bedossa, C. D. Guy, J. M. Schattenberg, R. Loomba, R. Taub, D. Labriola, S. E. Moussa, G. W. Neff, M. E. Rinella, Q. M. Anstee, M. F. Abdelmalek, Z. Younossi, S. J. Baum, S. Francque, M. R. Charlton, P. N. Newsome, N. Lanthier, I. Schiefke, A. Mangia, J. M. Pericàs, R. Patil, A. J. Sanyal, M. Noureddin, M. B. Bansal, N. Alkhouri, L. Castera, M. Rudraraju and V. Ratziu (2024). "A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis." N Engl J Med 390(6): 497-509.
Harrison, S. A., G. Neff, C. D. Guy, M. R. Bashir, A. H. Paredes, J. P. Frias, Z. Younes, J. F. Trotter, N. T. Gunn, S. E. Moussa, A. Kohli, K. Nelson, M. Gottwald, W. C. G. Chang, A. Z. Yan, A. M. DePaoli, L. Ling and H. D. Lieu (2021). "Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis." Gastroenterology 160(1): 219-231.e211.
Harrison, S. A., P. J. Ruane, B. L. Freilich, G. Neff, R. Patil, C. A. Behling, C. Hu, E. Fong, B. de Temple, E. J. Tillman, T. P. Rolph, A. Cheng and K. Yale (2021). "Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial." Nat Med 27(7): 1262-1271.
Harrison, S. A., R. Taub, G. W. Neff, K. J. Lucas, D. Labriola, S. E. Moussa, N. Alkhouri and M. R. Bashir (2023). "Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial." Nat Med(29(11)): 2919-2928.
Hashida, R., T. Kawaguchi, M. Bekki, M. Omoto, H. Matsuse, T. Nago, Y. Takano, T. Ueno, H. Koga, J. George, N. Shiba and T. Torimura (2017). "Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review." J Hepatol 66(1): 142-152.
Hayat, U., A. A. Siddiqui, H. Okut, S. Afroz, S. Tasleem and A. Haris (2021). "The effect of coffee consumption on the non-alcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies." Ann Hepatol 20: 100254.
Hofmann, W. P., P. Buggisch, L. Schubert, N. Dikopoulos, J. Schwenzer, M. Muche, G. Felten, R. Heyne, P. Ingiliz, A. Schmidt, K. Stein, H. Wedemeyer, T. Berg, J. Wiegand, F. Lammert, S. Zeuzem and J. M. Schattenberg (2020). "The Fatty Liver Assessment in Germany (FLAG) cohort study identifies large heterogeneity in NAFLD care." JHEP Rep 2(6): 100168.
Hohenester, S., S. Christiansen, J. Nagel, R. Wimmer, R. Artmann, G. Denk, M. Bischoff, G. Bischoff and C. Rust (2018). "Lifestyle intervention for morbid obesity: effects on liver steatosis, inflammation, and fibrosis." Am J Physiol Gastrointest Liver Physiol 315(3): G329-g338.
Huber, Y., A. Schulz, I. Schmidtmann, M. Beutel, N. Pfeiffer, T. Münzel, P. R. Galle, P. S. Wild, K. J. Lackner and J. M. Schattenberg (2022). "Prevalence and Risk Factors of Advanced Liver Fibrosis in a Population-Based Study in Germany." Hepatol Commun 6(6): 1457-1466.
Johnston, R. D., M. C. Stephenson, H. Crossland, S. M. Cordon, E. Palcidi, E. F. Cox, M. A. Taylor, G. P. Aithal and I. A. Macdonald (2013). "No difference between high-fructose and high-glucose diets on liver triacylglycerol or biochemistry in healthy overweight men." Gastroenterology 145(5): 1016-1025.e1012.
Kanwal, F., J. R. Kramer, S. Mapakshi, Y. Natarajan, M. Chayanupatkul, P. A. Richardson, L. Li, R. Desiderio, A. P. Thrift, S. M. Asch, J. Chu and H. B. El-Serag (2018). "Risk of Hepatocellular Cancer in Patients With Non-Alcoholic Fatty Liver Disease." Gastroenterology 155(6): 1828-1837.e1822.
Karlas, T., D. Petroff, M. Sasso, J. G. Fan, Y. Q. Mi, V. de Lédinghen, M. Kumar, M. Lupsor-Platon, K. H. Han, A. C. Cardoso, G. Ferraioli, W. K. Chan, V. W. Wong, R. P. Myers, K. Chayama, M. Friedrich-Rust, M. Beaugrand, F. Shen, J. B. Hiriart, S. K. Sarin, R. Badea, K. S. Jung, P. Marcellin, C. Filice, S. Mahadeva, G. L. Wong, P. Crotty, K. Masaki, J. Bojunga, P. Bedossa, V. Keim and J. Wiegand (2017). "Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis." J Hepatol 66(5): 1022-1030.
Kim, G. A., H. C. Lee, J. Choe, M. J. Kim, M. J. Lee, H. S. Chang, I. Y. Bae, H. K. Kim, J. An, J. H. Shim, K. M. Kim and Y. S. Lim (2017). "Association between non-alcoholic fatty liver disease and cancer incidence rate." J Hepatol. 2017 Nov 2:S0168-8278(17)32294-8.
Kim, Y., Y. Cho, Y. H. Lee, D. H. Seo, S. H. Ahn, S. Hong and S. H. Kim (2025). "Association of lean metabolic dysfunction-associated steatotic liver disease with carotid plaque progression in patients with type 2 diabetes mellitus." J Diabetes Investig Sep(16(9)): 1713-1719.
Kleiner, D. E., E. M. Brunt, M. Van Natta, C. Behling, M. J. Contos, O. W. Cummings, L. D. Ferrell, Y. C. Liu, M. S. Torbenson, A. Unalp-Arida, M. Yeh, A. J. McCullough, A. J. Sanyal and N. Nonalcoholic Steatohepatitis Clinical Research (2005). "Design and validation of a histological scoring system for nonalcoholic fatty liver disease." Hepatology 41(6): 1313-1321.
Krawczyk, M., R. Liebe and F. Lammert (2020). "Toward Genetic Prediction of Nonalcoholic Fatty Liver Disease Trajectories: PNPLA3 and Beyond." Gastroenterology 158(7): 1865-1880.e1861.
Krawczyk, M., M. Rau, J. M. Schattenberg, H. Bantel, A. Pathil, M. Demir, J. Kluwe, T. Boettler, F. Lammert and A. Geier (2017). "Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study." J Lipid Res 58(1): 247-255.
Labenz, C., Y. Huber, E. Kalliga, M. Nagel, C. Ruckes, B. K. Straub, P. R. Galle, M. A. Wörns, Q. M. Anstee, D. Schuppan and J. M. Schattenberg (2018). "Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany." Aliment Pharmacol Ther 48(10): 1109-1116.
LaBrecque, D. R., Z. Abbas, F. Anania, P. Ferenci, A. G. Khan, K. L. Goh, S. S. Hamid, V. Isakov, M. Lizarzabal, M. M. Peñaranda, J. F. Ramos, S. Sarin, D. Stimac, A. B. Thomson, M. Umar, J. Krabshuis and A. LeMair (2014). "World Gastroenterology Organisation global guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis." J Clin Gastroenterol 48(6): 467-473.
Le, M. H., D. M. Le, T. C. Baez, Y. Wu, T. Ito, E. Y. Lee, K. Lee, C. D. Stave, L. Henry, S. D. Barnett, R. Cheung and M. H. Nguyen (2023). "Global incidence of non-alcoholic fatty liver disease: A systematic review and meta-analysis of 63 studies and 1,201,807 persons." J Hepatol 79(2): 287-295.
Lee, B. P., J. L. Dodge and N. A. Terrault (2023). "National prevalence estimates for steatotic liver disease and Sub-Classifications using consensus nomenclature." Hepatology. 2024 Mar 1;79(3):666-673.
Lin, J., Y. Huang, B. Xu, X. Gu, J. Huang, J. Sun, L. Jia, J. He, C. Huang, X. Wei, J. Chen, X. Chen, J. Zhou, L. Wu, P. Zhang, Y. Zhu, H. Xia, G. Wen, Y. Liu, S. Liu, Y. Zeng, L. Zhou, H. Jia, H. He, Y. Xue, F. Wu and H. Zhang (2025). "Effect of dapagliflozin on metabolic dysfunction-associated steatohepatitis: multicentre, double blind, randomised, placebo controlled trial." Bmj 389: e083735.
Long, M. T., X. Zhang, H. Xu, C. T. Liu, K. E. Corey, R. T. Chung, R. Loomba and E. J. Benjamin (2021). "Hepatic Fibrosis Associates With Multiple Cardiometabolic Disease Risk Factors: The Framingham Heart Study." Hepatology 73(2): 548-559.
Loomba, R. (2018). "Role of imaging-based biomarkers in NAFLD: Recent advances in clinical application and future research directions." J Hepatol 68(2): 296-304.
Loomba, R. and L. A. Adams (2020). "Advances in non-invasive assessment of hepatic fibrosis." Gut 69(7): 1343-1352.
Loomba, R., S. L. Friedman and G. I. Shulman (2021). "Mechanisms and disease consequences of nonalcoholic fatty liver disease." Cell 184(10): 2537-2564.
Loomba, R., M. L. Hartman, E. J. Lawitz, R. Vuppalanchi, J. Boursier, E. Bugianesi, M. Yoneda, C. Behling, O. W. Cummings, Y. Tang, B. Brouwers, D. A. Robins, A. Nikooie, M. C. Bunck, A. Haupt and A. J. Sanyal (2024). "Tirzepatide for Metabolic Dysfunction-Associated Steatohepatitis with Liver Fibrosis." N Engl J Med 391(4): 299-310.
Loomba, R., J. K. Lim, H. Patton and H. B. El-Serag (2020). "AGA Clinical Practice Update on Screening and Surveillance for Hepatocellular Carcinoma in Patients With Nonalcoholic Fatty Liver Disease: Expert Review." Gastroenterology 158(6): 1822-1830.
Loomba, R., R. Mohseni, K. J. Lucas, J. A. Gutierrez, R. G. Perry, J. F. Trotter, R. S. Rahimi, S. A. Harrison, V. Ajmera, J. D. Wayne, M. O'Farrell, W. McCulloch, K. Grimmer, M. Rinella, V. Wai-Sun Wong, V. Ratziu, G. J. Gores, B. A. Neuschwander-Tetri and G. Kemble (2021). "TVB-2640 (FASN Inhibitor) for the Treatment of Nonalcoholic Steatohepatitis: FASCINATE-1, a Randomized, Placebo-Controlled Phase 2a Trial." Gastroenterology 161(5): 1475-1486.
Loomba, R., M. Noureddin, K. V. Kowdley, A. Kohli, A. Sheikh, G. Neff, B. R. Bhandari, N. Gunn, S. H. Caldwell, Z. Goodman, I. Wapinski, M. Resnick, A. H. Beck, D. Ding, C. Jia, J. C. Chuang, R. S. Huss, C. Chung, G. M. Subramanian, R. P. Myers, K. Patel, B. B. Borg, R. Ghalib, H. Kabler, J. Poulos, Z. Younes, M. Elkhashab, T. Hassanein, R. Iyer, P. Ruane, M. L. Shiffman, S. Strasser, V. W. Wong and N. Alkhouri (2021). "Combination Therapies Including Cilofexor and Firsocostat for Bridging Fibrosis and Cirrhosis Attributable to NASH." Hepatology 73(2): 625-643.
Loomba, R., A. J. Sanyal, K. V. Kowdley, D. L. Bhatt, N. Alkhouri, J. P. Frias, P. Bedossa, S. A. Harrison, D. Lazas, R. Barish, M. D. Gottwald, S. Feng, G. D. Agollah, C. L. Hartsfield, H. Mansbach, M. Margalit and M. F. Abdelmalek (2023). "Randomized, Controlled Trial of the FGF21 Analogue Pegozafermin in NASH." N Engl J Med 389(11): 998-1008.
Loosen, S. H., K. Kostev, V. Keitel, F. Tacke, C. Roderburg and T. Luedde (2022). "An elevated FIB-4 score predicts liver cancer development: A longitudinal analysis from 29,999 patients with NAFLD." J Hepatol 76(1): 247-248.
Ludwig, J., T. R. Viggiano, D. B. McGill and B. J. Oh (1980). "Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease." Mayo Clin Proc 55(7): 434-438.
Markova, M., O. Pivovarova, S. Hornemann, S. Sucher, T. Frahnow, K. Wegner, J. Machann, K. J. Petzke, J. Hierholzer, R. Lichtinghagen, C. Herder, M. Carstensen-Kirberg, M. Roden, N. Rudovich, S. Klaus, R. Thomann, R. Schneeweiss, S. Rohn and A. F. Pfeiffer (2017). "Isocaloric Diets High in Animal or Plant Protein Reduce Liver Fat and Inflammation in Individuals With Type 2 Diabetes." Gastroenterology 152(3): 571-585.e578.
Mofidi, F., H. Poustchi, Z. Yari, B. Nourinayyer, S. Merat, M. Sharafkhah, R. Malekzadeh and A. Hekmatdoost (2017). "Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial." Br J Nutr 117(5): 662-668.
Morita, Y., T. Ueno, N. Sasaki, K. Kuhara, S. Yoshioka, Y. Tateishi, E. Nagata, M. Kage and M. Sata (2005). "Comparison of liver histology between patients with non-alcoholic steatohepatitis and patients with alcoholic steatohepatitis in Japan." Alcohol Clin Exp Res 29(12 Suppl): 277S-281S.
Mózes, F. E., J. A. Lee, E. A. Selvaraj, A. N. A. Jayaswal, M. Trauner, J. Boursier, C. Fournier, K. Staufer, R. E. Stauber, E. Bugianesi, R. Younes, S. Gaia, M. Lupșor-Platon, S. Petta, T. Shima, T. Okanoue, S. Mahadeva, W. K. Chan, P. J. Eddowes, G. M. Hirschfield, P. N. Newsome, V. W. Wong, V. de Ledinghen, J. Fan, F. Shen, J. F. Cobbold, Y. Sumida, A. Okajima, J. M. Schattenberg, C. Labenz, W. Kim, M. S. Lee, J. Wiegand, T. Karlas, Y. Yılmaz, G. P. Aithal, N. Palaniyappan, C. Cassinotto, S. Aggarwal, H. Garg, G. J. Ooi, A. Nakajima, M. Yoneda, M. Ziol, N. Barget, A. Geier, T. Tuthill, M. J. Brosnan, Q. M. Anstee, S. Neubauer, S. A. Harrison, P. M. Bossuyt and M. Pavlides (2022). "Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: an individual patient data meta-analysis." Gut 71(5): 1006-1019.
Nasr, P., S. Ignatova, S. Kechagias and M. Ekstedt (2018). "Natural history of nonalcoholic fatty liver disease: A prospective follow-up study with serial biopsies." Hepatol Commun 2(2): 199-210.
Newsome, P. N. and P. Ambery (2023). "Incretins (GLP-1 receptor agonists and dual/triple agonists) and the liver." J Hepatol 79(6): 1557-1565.
Noureddin, M., J. P. Frias, G. W. Neff, K. J. Lucas, C. Behling, P. Bedossa, J. Dubourg, D. Chan, M. Burch, E. Fong, B. de Temple, M. Minerva, K. Barrett, R. Shringarpure, E. J. Tillman, T. Rolph, A. Cheng and K. Yale (2025). "Safety and efficacy of once-weekly efruxifermin versus placebo in metabolic dysfunction-associated steatohepatitis (HARMONY): 96-week results from a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial." Lancet 406(10504): 719-730.
Ooi, G. J., S. Mgaieth, G. D. Eslick, P. R. Burton, W. W. Kemp, S. K. Roberts and W. A. Brown (2018). "Systematic review and meta-analysis: non-invasive detection of non-alcoholic fatty liver disease related fibrosis in the obese." Obes Rev 19(2): 281-294.
Paik, J. M., L. Henry, Y. Younossi, J. Ong, S. Alqahtani and Z. M. Younossi (2023). "The burden of nonalcoholic fatty liver disease (NAFLD) is rapidly growing in every region of the world from 1990 to 2019." Hepatol Commun 7(10).
Park, H., T. Cheuk-Fung Yip, E. L. Yoon, G. Lai-Hung Wong, H. S. Lee, V. Wai-Sun Wong, J. Che-To Lai and D. W. Jun (2025). "Impact of cardiometabolic risk factors on hepatic fibrosis and clinical outcomes in MASLD: A population-based multi-cohort study." JHEP Rep 7(6): 101388.
Parry, S. A. and L. Hodson (2020). "Managing NAFLD in Type 2 Diabetes: The Effect of Lifestyle Interventions, a Narrative Review." Adv Ther 37(4): 1381-1406.
Patel, K., S. A. Harrison, M. Elkhashab, J. F. Trotter, R. Herring, S. E. Rojter, Z. Kayali, V. W. Wong, S. Greenbloom, S. Jayakumar, M. L. Shiffman, B. Freilich, E. J. Lawitz, E. J. Gane, E. Harting, J. Xu, A. N. Billin, C. Chung, C. S. Djedjos, G. M. Subramanian, R. P. Myers, M. S. Middleton, M. Rinella and M. Noureddin (2020). "Cilofexor, a Nonsteroidal FXR Agonist, in Patients With Noncirrhotic NASH: A Phase 2 Randomized Controlled Trial." Hepatology 72(1): 58-71.
Poole, R., O. J. Kennedy, P. Roderick, J. A. Fallowfield, P. C. Hayes and J. Parkes (2017). "Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes." Bmj 359: j5024.
Rademacher, S., N. F. Aehling, R. Sucher, T. Berg and D. Seehofer (2021). "Current State and Future Possibilities in Liver Transplantation." Surg Technol Int 39: 128-134.
Ratziu, V., L. de Guevara, R. Safadi, F. Poordad, F. Fuster, J. Flores-Figueroa, M. Arrese, A. L. Fracanzani, D. Ben Bashat, K. Lackner, T. Gorfine, S. Kadosh, R. Oren, M. Halperin, L. Hayardeny, R. Loomba, S. Friedman and A. J. Sanyal (2021). "Aramchol in patients with nonalcoholic steatohepatitis: a randomized, double-blind, placebo-controlled phase 2b trial." Nat Med 27(10): 1825-1835.
Ratziu, V., P. Giral, F. Charlotte, E. Bruckert, V. Thibault, I. Theodorou, L. Khalil, G. Turpin, P. Opolon and T. Poynard (2000). "Liver fibrosis in overweight patients." Gastroenterology 118(6): 1117-1123.
Rau, M. and A. Geier (2021). "An update on drug development for the treatment of nonalcoholic fatty liver disease - from ongoing clinical trials to future therapy." Expert Rev Clin Pharmacol 14(3): 333-340.
Rau, M., A. Rehman, M. Dittrich, A. K. Groen, H. M. Hermanns, F. Seyfried, N. Beyersdorf, T. Dandekar, P. Rosenstiel and A. Geier (2018). "Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease." United European Gastroenterol J 6(10): 1496-1507.
Rich, N. E., S. Oji, A. R. Mufti, J. D. Browning, N. D. Parikh, M. Odewole, H. Mayo and A. G. Singal (2018). "Racial and Ethnic Disparities in Nonalcoholic Fatty Liver Disease Prevalence, Severity, and Outcomes in the United States: A Systematic Review and Meta-analysis." Clin Gastroenterol Hepatol 16(2): 198-210.e192.
Rinella, M. E., J. V. Lazarus, V. Ratziu, S. M. Francque, A. J. Sanyal, F. Kanwal, D. Romero, M. F. Abdelmalek, Q. M. Anstee, J. P. Arab, M. Arrese, R. Bataller, U. Beuers, J. Boursier, E. Bugianesi, C. D. Byrne, G. E. Castro Narro, A. Chowdhury, H. Cortez-Pinto, D. R. Cryer, K. Cusi, M. El-Kassas, S. Klein, W. Eskridge, J. Fan, S. Gawrieh, C. D. Guy, S. A. Harrison, S. U. Kim, B. G. Koot, M. Korenjak, K. V. Kowdley, F. Lacaille, R. Loomba, R. Mitchell-Thain, T. R. Morgan, E. E. Powell, M. Roden, M. Romero-Gómez, M. Silva, S. P. Singh, S. C. Sookoian, C. W. Spearman, D. Tiniakos, L. Valenti, M. B. Vos, V. W. Wong, S. Xanthakos, Y. Yilmaz, Z. Younossi, A. Hobbs, M. Villota-Rivas and P. N. Newsome (2023). "A multisociety Delphi consensus statement on new fatty liver disease nomenclature." J Hepatol. 2023 Dec;79(6):1542-1556.
Roeb, E. (2021). "Excess Body Weight and Metabolic (Dysfunction)-Associated Fatty Liver Disease (MAFLD)." Visceral Medicine. 2022 Apr;38(2):126-132.
Roeb, E., A. Canbay, H. Bantel, J. Bojunga, J. de Laffolie, M. Demir, U. W. Denzer, A. Geier, W. P. Hofmann, C. Hudert, T. Karlas, M. Krawczyk, T. Longerich, T. Lüdde, M. Roden, J. M. Schattenberg, M. Sterneck, A. Tannapfel, P. Lorenz and F. Tacke (2024). "Amendment „Neue Nomenklatur zur MASLD (Metabolic Dysfunction Associated Steatotic Liver Disease; metabolische Dysfunktion assoziierte steatotische Lebererkrankung)“ zur S2k-Leitlinie „Nicht-alkoholische Fettlebererkrankung“ (v.2.0/April 2022) der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) " Z Gastroenterol 62(7): 1077-1087.
Roeb, E., A. Canbay, H. Bantel, J. Bojunga, J. de Laffolie, M. Demir, U. W. Denzer, A. Geier, W. P. Hofmann, C. Hudert, T. Karlas, M. Krawczyk, T. Longerich, T. Luedde, M. Roden, J. Schattenberg, M. Sterneck, A. Tannapfel, P. Lorenz and F. Tacke (2022). "Aktualisierte S2k-Leitlinie nicht-alkoholische Fettlebererkrankung der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) – April 2022 – AWMF-Registernummer: 021–025." Z Gastroenterol 60(9): 1346-1421.
Romeo, S., J. Kozlitina, C. Xing, A. Pertsemlidis, D. Cox, L. A. Pennacchio, E. Boerwinkle, J. C. Cohen and H. H. Hobbs (2008). "Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease." Nat Genet 40(12): 1461-1465.
Saadeh, S., Z. M. Younossi, E. M. Remer, T. Gramlich, J. P. Ong, M. Hurley, K. D. Mullen, J. N. Cooper and M. J. Sheridan (2002). "The utility of radiological imaging in nonalcoholic fatty liver disease." Gastroenterology 123(3): 745-750.
Sanyal, A. J., S. A. Harrison, V. Ratziu, M. F. Abdelmalek, A. M. Diehl, S. Caldwell, M. L. Shiffman, R. Aguilar Schall, C. Jia, B. McColgan, C. S. Djedjos, J. G. McHutchison, G. M. Subramanian, R. P. Myers, Z. Younossi, A. J. Muir, N. H. Afdhal, J. Bosch and Z. Goodman (2019). "The Natural History of Advanced Fibrosis Due to Nonalcoholic Steatohepatitis: Data From the Simtuzumab Trials." Hepatology 70(6): 1913-1927.
Sanyal, A. J., P. Lopez, E. J. Lawitz, K. J. Lucas, J. Loeffler, W. Kim, G. B. B. Goh, J. F. Huang, C. Serra, P. Andreone, Y. C. Chen, S. H. Hsia, V. Ratziu, D. Aizenberg, H. Tobita, A. M. Sheikh, J. M. Vierling, Y. J. Kim, H. Hyogo, D. Tai, Z. Goodman, F. Schaefer, I. R. I. Carbarns, S. Lamle, M. Martic, N. V. Naoumov and C. A. Brass (2023). "Tropifexor for nonalcoholic steatohepatitis: an adaptive, randomized, placebo-controlled phase 2a/b trial." Nat Med 29(2): 392-400.
Sanyal, A. J., P. N. Newsome, I. Kliers, L. H. Østergaard, M. T. Long, M. S. Kjær, A. M. G. Cali, E. Bugianesi, M. E. Rinella, M. Roden and V. Ratziu (2025). "Phase 3 Trial of Semaglutide in Metabolic Dysfunction-Associated Steatohepatitis." N Engl J Med 392(21): 2089-2099.
Sanyal, A. J., V. Ratziu, R. Loomba, Q. M. Anstee, K. V. Kowdley, M. E. Rinella, M. Y. Sheikh, J. F. Trotter, W. Knapple, E. J. Lawitz, M. F. Abdelmalek, P. N. Newsome, J. Boursier, P. Mathurin, J. F. Dufour, M. M. Berrey, S. J. Shiff, S. Sawhney, T. Capozza, R. Leyva, S. A. Harrison and Z. M. Younossi (2023). "Results from a new efficacy and safety analysis of the REGENERATE trial of obeticholic acid for treatment of pre-cirrhotic fibrosis due to non-alcoholic steatohepatitis." J Hepatol 79(5): 1110-1120.
Schmid, A., M. Arians, T. Karrasch, J. Pons-Kühnemann, A. Schäffler, M. Roderfeld and E. Roeb (2022). "Improvement of Type 2 Diabetes Mellitus and Attenuation of NAFLD Are Associated with the Success of Obesity Therapy." J Clin Med 11(7).
Scorletti, E., P. R. Afolabi, E. A. Miles, D. E. Smith, A. Almehmadi, A. Alshathry, C. E. Childs, S. Del Fabbro, J. Bilson, H. E. Moyses, G. F. Clough, J. K. Sethi, J. Patel, M. Wright, D. J. Breen, C. Peebles, A. Darekar, R. Aspinall, A. J. Fowell, J. K. Dowman, V. Nobili, G. Targher, N. M. Delzenne, L. B. Bindels, P. C. Calder and C. D. Byrne (2020). "Synbiotics Alter Fecal Microbiomes, But Not Liver Fat or Fibrosis, in a Randomized Trial of Patients With Nonalcoholic Fatty Liver Disease." Gastroenterology 158(6): 1597-1610.e1597.
Serra-Burriel, M., A. Juanola, F. Serra-Burriel, M. Thiele, I. Graupera, E. Pose, G. Pera, I. Grgurevic, L. Caballeria, S. Piano, L. van Kleef, M. Reichert, D. Roulot, J. M. Pericàs, J. M. Schattenberg, E. A. Tsochatztis, I. N. Guha, M. Garcia-Retortillo, R. Hernández, J. Hoyo, M. Fuentes, C. Expósito, A. Martínez, P. Such, A. Madir, S. Detlefsen, M. Tonon, A. Martini, A. T. Ma, J. Pich, E. Bonfill, M. Juan, A. Soria, M. Carol, J. Gratacós-Ginès, R. M. Morillas, P. Toran, J. M. Navarrete, A. Torrejón, C. Fournier, A. Llorca, A. Arslanow, H. J. de Koning, F. Cucchietti, M. Manns, P. N. Newsome, R. Hernáez, A. Allen, P. Angeli, R. J. de Knegt, T. H. Karlsen, P. Galle, V. W. Wong, N. Fabrellas, L. Castera, A. Krag, F. Lammert, P. S. Kamath and P. Ginès (2023). "Development, validation, and prognostic evaluation of a risk score for long-term liver-related outcomes in the general population: a multicohort study." Lancet 402(10406): 988-996.
Shen, H., S. Lipka, A. Kumar and P. Mustacchia (2014). "Association between nonalcoholic fatty liver disease and colorectal adenoma: a systemic review and meta-analysis." J Gastrointest Oncol 5(6): 440-446.
Singh, S., A. M. Allen, Z. Wang, L. J. Prokop, M. H. Murad and R. Loomba (2015). "Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies." Clin Gastroenterol Hepatol 13(4): 643-654.e641-649; quiz e639-640.
Stricker, S., S. Rudloff, A. Geier, A. Steveling, E. Roeb and K. P. Zimmer (2021). "Fructose Consumption-Free Sugars and Their Health Effects." Dtsch Arztebl Int 118(5): 71-78.
Suárez, M., N. Boqué, J. M. Del Bas, J. Mayneris-Perxachs, L. Arola and A. Caimari (2017). "Mediterranean Diet and Multi-Ingredient-Based Interventions for the Management of Non-Alcoholic Fatty Liver Disease." Nutrients 9(10).
Tacke, F., D. C. Kroy, A. P. Barreiros and U. P. Neumann (2016). "Liver transplantation in Germany." Liver Transpl 22(8): 1136-1142.
Trépo, E. and L. Valenti (2020). "Update on NAFLD genetics: From new variants to the clinic." J Hepatol 72(6): 1196-1209.
Verrastro, O., S. Panunzi, L. Castagneto-Gissey, A. De Gaetano, E. Lembo, E. Capristo, C. Guidone, G. Angelini, F. Pennestrì, L. Sessa, F. M. Vecchio, L. Riccardi, M. A. Zocco, I. Boskoski, J. R. Casella-Mariolo, P. Marini, M. Pompili, G. Casella, E. Fiori, F. Rubino, S. R. Bornstein, M. Raffaelli and G. Mingrone (2023). "Bariatric-metabolic surgery versus lifestyle intervention plus best medical care in non-alcoholic steatohepatitis (BRAVES): a multicentre, open-label, randomised trial." Lancet 401(10390): 1786-1797.
Vilar-Gomez, E., L. Calzadilla-Bertot, V. Wai-Sun Wong, M. Castellanos, R. Aller-de la Fuente, M. Metwally, M. Eslam, L. Gonzalez-Fabian, M. Alvarez-Quiñones Sanz, A. F. Conde-Martin, B. De Boer, D. McLeod, A. W. Hung Chan, N. Chalasani, J. George, L. A. Adams and M. Romero-Gomez (2018). "Fibrosis Severity as a Determinant of Cause-Specific Mortality in Patients With Advanced Nonalcoholic Fatty Liver Disease: A Multi-National Cohort Study." Gastroenterology 155(2): 443-457.e417.
Vilar-Gomez, E., L. Calzadilla-Bertot, V. W. Wong, M. Castellanos, R. Aller-de la Fuente, M. Eslam, G. L. Wong, J. George, M. Romero-Gomez and L. A. Adams (2021). "Type 2 Diabetes and Metformin Use Associate With Outcomes of Patients With Nonalcoholic Steatohepatitis-Related, Child-Pugh A Cirrhosis." Clin Gastroenterol Hepatol 19(1): 136-145.e136.
Vilar-Gomez, E., Y. Martinez-Perez, L. Calzadilla-Bertot, A. Torres-Gonzalez, B. Gra-Oramas, L. Gonzalez-Fabian, S. L. Friedman, M. Diago and M. Romero-Gomez (2015). "Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis." Gastroenterology 149(2): 367-378.e365; quiz e314-365.
Williams, C. D., J. Stengel, M. I. Asike, D. M. Torres, J. Shaw, M. Contreras, C. L. Landt and S. A. Harrison (2011). "Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study." Gastroenterology 140(1): 124-131.
Wong, V. W., G. L. Wong, R. S. Chan, S. S. Shu, B. H. Cheung, L. S. Li, A. M. Chim, C. K. Chan, J. K. Leung, W. C. Chu, J. Woo and H. L. Chan (2018). "Beneficial effects of lifestyle intervention in non-obese patients with non-alcoholic fatty liver disease." J Hepatol 69(6): 1349-1356.
Xiao, G., S. Zhu, X. Xiao, L. Yan, J. Yang and G. Wu (2017). "Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: A meta-analysis." Hepatology 66(5): 1486-1501.
Xiao, S., Y. Liu, X. Fu, T. Chen and W. Xie (2024). "Modifiable Risk Factors for Hepatocellular Carcinoma in Patients with Metabolic Dysfunction-Associated Steatotic Liver Disease: A Meta-Analysis." Am J Med 137(11): 1072-1081.e1032.
Yang, Y., Y. Teng, J. Shi, J. Xu, J. Bao, X. Zhang and Q. Wang (2023). "Association of nonalcoholic fatty liver disease with colorectal adenomatous polyps and non-adenomatous polyps: a cross-sectional study." Eur J Gastroenterol Hepatol 35(12): 1389-1393.
Ye, Q., B. Zou, Y. H. Yeo, J. Li, D. Q. Huang, Y. Wu, H. Yang, C. Liu, L. Y. Kam, X. X. E. Tan, N. Chien, S. Trinh, L. Henry, C. D. Stave, T. Hosaka, R. C. Cheung and M. H. Nguyen (2020). "Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis." Lancet Gastroenterol Hepatol 5(8): 739-752.
Young, S., R. Tariq, J. Provenza, S. K. Satapathy, K. Faisal, A. Choudhry, S. L. Friedman and A. K. Singal (2020). "Prevalence and Profile of Nonalcoholic Fatty Liver Disease in Lean Adults: Systematic Review and Meta-Analysis." Hepatol Commun 4(7): 953-972.
Younossi, Z., Q. M. Anstee, M. Marietti, T. Hardy, L. Henry, M. Eslam, J. George and E. Bugianesi (2018). "Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention." Nat Rev Gastroenterol Hepatol 15(1): 11-20.
Younossi, Z., P. Golabi, J. Paik, A. Henry, C. Van Dongen and L. Henry (2023). "The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH): a systematic review." Hepatology. 2023 Apr 1;77(4):1335-1347.
Younossi, Z. M. (2019). "Non-alcoholic fatty liver disease - A global public health perspective." J Hepatol 70(3): 531-544.
Younossi, Z. M., L. Henry, H. Bush and A. Mishra (2018). "Clinical and Economic Burden of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis." Clin Liver Dis 22(1): 1-10.
Younossi, Z. M., A. B. Koenig, D. Abdelatif, Y. Fazel, L. Henry and M. Wymer (2016). "Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes." Hepatology 64(1): 73-84.
Younossi, Z. M., R. Loomba, Q. M. Anstee, M. E. Rinella, E. Bugianesi, G. Marchesini, B. A. Neuschwander-Tetri, L. Serfaty, F. Negro, S. H. Caldwell, V. Ratziu, K. E. Corey, S. L. Friedman, M. F. Abdelmalek, S. A. Harrison, A. J. Sanyal, J. E. Lavine, P. Mathurin, M. R. Charlton, Z. D. Goodman, N. P. Chalasani, K. V. Kowdley, J. George and K. Lindor (2018). "Diagnostic modalities for nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, and associated fibrosis." Hepatology 68(1): 349-360.
Younossi, Z. M., J. M. Paik, L. Henry, J. Yang, G. Fernandes, M. Stepanova and F. Nader (2023). "The Growing Economic and Clinical Burden of Nonalcoholic Steatohepatitis (NASH) in the United States." J Clin Exp Hepatol 13(3): 454-467.
Younossi, Z. M., V. Ratziu, R. Loomba, M. Rinella, Q. M. Anstee, Z. Goodman, P. Bedossa, A. Geier, S. Beckebaum, P. N. Newsome, D. Sheridan, M. Y. Sheikh, J. Trotter, W. Knapple, E. Lawitz, M. F. Abdelmalek, K. V. Kowdley, A. J. Montano-Loza, J. Boursier, P. Mathurin, E. Bugianesi, G. Mazzella, A. Olveira, H. Cortez-Pinto, I. Graupera, D. Orr, L. L. Gluud, J. F. Dufour, D. Shapiro, J. Campagna, L. Zaru, L. MacConell, R. Shringarpure, S. Harrison and A. J. Sanyal (2019). "Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial." Lancet 394(10215): 2184-2196.
Younossi, Z. M., M. Stepanova, J. Ong, G. Trimble, S. AlQahtani, I. Younossi, A. Ahmed, A. Racila and L. Henry (2021). "Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States." Clin Gastroenterol Hepatol 19(3): 580-589.e585.
Younossi, Z. M., S. Zelber-Sagi, J. V. Lazarus, V. W. Wong, Y. Yilmaz, A. Duseja, Y. Eguchi, L. Castera, M. G. Pessoa, C. P. Oliveira, M. El-Kassas, E. Tsochatzis, J. G. Fan, C. W. Spearman, F. Tacke, M. I. Castellanos Fernandez, N. Alkhouri, J. M. Schattenberg, M. Romero-Gómez, M. Noureddin, A. M. Allen, J. P. Ong, S. K. Roberts, J. H. Shubrook, P. Burra, R. Kohli, A. Kautz, A. G. Holleboom, B. Lam, S. Isaacs, P. Macedo, A. Gastaldelli, L. Henry, D. Ivancovsky-Wajcman, F. Nader, L. de Avila, J. K. Price, H. E. Mark, M. Villota-Rivas, A. Barberá, M. Kalligeros, L. H. Gerber and S. A. Alqahtani (2025). "Global Consensus Recommendations for Metabolic Dysfunction-Associated Steatotic Liver Disease and Steatohepatitis." Gastroenterology Apr (11): S0016-5085(0025)00632-00638.
Yuan, X., D. Waterworth, J. R. Perry, N. Lim, K. Song, J. C. Chambers, W. Zhang, P. Vollenweider, H. Stirnadel, T. Johnson, S. Bergmann, N. D. Beckmann, Y. Li, L. Ferrucci, D. Melzer, D. Hernandez, A. Singleton, J. Scott, P. Elliott, G. Waeber, L. Cardon, T. M. Frayling, J. S. Kooner and V. Mooser (2008). "Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes." Am J Hum Genet 83(4): 520-528.
Zachou, M., P. Flevari, N. Nasiri-Ansari, C. Varytimiadis, E. Kalaitzakis, E. Kassi and T. Androutsakos (2024). "The role of anti-diabetic drugs in NAFLD. Have we found the Holy Grail? A narrative review." Eur J Clin Pharmacol 80(1): 127-150.
Zimmer, V. and F. Lammert (2011). "Genetics and epigenetics in the fibrogenic evolution of chronic liver diseases." Best Pract Res Clin Gastroenterol 25(2): 269-280.
