8. Grading and staging of liver diseases
Michael Praktiknjo, Juliana Gödiker
Liver disease encompasses a spectrum of disorders, with cirrhosis and portal hypertension representing critical and advanced stages that necessitate precise grading and staging for effective clinical management. Cirrhosis, characterised by the progressive replacement of healthy liver tissue with scar tissue, fundamentally alters liver architecture and function, often leading to severe complications. Among these complications, portal hypertension—the increased pressure within the portal venous system—stands out as a major driver of morbidity and mortality. Accurate assessment of the severity and progression of liver disease is crucial for prognosis, therapeutic decision-making, and evaluation of treatment efficacy.
The current Baveno VII consensus promotes the use of non-invasive methods to assess clinically significant portal hypertension (CSPH), aiming to identify at-risk patients and reduce the need for unnecessary endoscopic screenings (de Franchis 2022). Additionally, spleen stiffness measurement (SSM) is gaining traction as a new elastography technique. Both elastography and cross-sectional imaging techniques now offer comparable predictive accuracy, and their effectiveness is enhanced when these non-invasive tests are used sequentially.
Nevertheless, the use of interventional transjugular procedures plays an increasingly relevant role in in the diagnosis of acute and chronic liver diseases. Measurement of the hepatic venous pressure gradient (HVPG) has become a relevant tool in clinical hepato y as it is considered the gold standard for sinusoidal portal hypertension (PH) diagnosis in patients with compensated advanced chronic liver disease (cACLD; compensated cirrhosis) according to the current Baveno VII consensus (de Franchis 2022). In addition, as HVPG measurement can be combined with transjugular liver biopsy (TJLB), the combination of these two procedures enables correlation of hemodynamic data with the underlying histopatho ical changes, providing a more comprehensive understanding of the pathophysio ical mechanisms of the underlying liver disease. More recently, endoscopic ultrasound (EUS-)guided approaches to obtaining liver biopsies and measurements of portal pressure gradient (EUS-PPG) gain attention as an emerging technique, overcoming most of the shortcomings of aforementioned HVPG measurements (Laleman 2023).
Clinical stages of liver cirrhosis
The progression of liver cirrhosis can be classified into different clinical stages. From compensated liver cirrhosis various events can lead to acute decompensation (AD) being defined by the sudden onset of ascites, hepatic encephalopathy, gastrointestinal hemorrhage, bacterial infections, or any combination of these conditions. At this stage, patients are highly susceptible to bacterial infections due to complex cirrhosis-associated immune dysfunction severely affecting the overall prognosis (Trebicka 2020). The initial occurrence of AD indicates a shift from compensated to decompensated cirrhosis. For decompensated cirrhosis the prognosis worsens significantly compared to compensated stages with a median survival of only about two years (D’Amico 2018). Modern concepts also include non-acute decompensation (NAD) defined as non-acute occurrence of grade 2 ascites and/or grade 1–2 HE manageable in the outpatient clinic, which have a better prognosis than AD (Schulz 2025, Tonon 2024). Decompensated cirrhosis is further identified by repeated episodes of AD, finally leading to acute-on-chronic liver failure (ACLF) (European Association for the Study of the Liver 2018, Moreau 2013).
Acute-on-chronic liver failure (ACLF) is a severe complication of liver cirrhosis and can occur in all of the disease stages of liver cirrhosis. ACLF is marked by a high short-term mortality (D’Amico 2018, Trebicka 2020). A bacterial infection, active consumption of alcohol and surgeries are only some factors that can trigger the development of an ACLF. However, in a significant number of patients the trigger cannot be identified (Trebicka 2021). For the definition of ACLF two criteria have to be fulfilled: presence of decompensated liver cirrhosis (in this case presence of ascites, bacterial infection, gastro-oesophageal bleeding or hepatic encephalopathy) and development of at least one organ failure (Table 1). The ACLF can be further divided into different grades: ACLF grade 1 (presence of renal failure alone or other organ failure in combination with renal dysfunction or hepatic encephalopathy), ACLF grade 2 (presence of two organ failures) and ACLF grade 3 (presence of at least three organ failures) (Arroyo 2020, European Association for the Study of the Liver 2023; Moreau 2013) (Table 2).
Table 1. Organ failure score in acute-on-chronic liver failure| Organ System | 1 Point | 2 Point | 3 Points |
| Liver (bilirubin, mg/dL) | Bilirubin <6 mg/dL | Bilirubin 6.0–11.9 mg/dL | Bilirubin ≥12 mg/dL |
| Kidney (creatinine, mg/dL) | Creatinine <1.5 mg/dL or 1.5–1.9 mg/dL | Creatinine 2.0–3.4 mg/dL | Creatinine ≥3.5 mg/dL or RRT |
| Brain (West Haven criteria) | Grade 0 | Grade 1–2 | Grade 3–4 |
| Coagulation (INR) | INR <2.0 | INR 2.0–2.4 | INR ≥2.5 |
| Circulation (MAP, mm Hg) | MAP ≥70 mm Hg | MAP <70 mm Hg | Vasopressor requirement |
| Respiration (PaO₂/FiO₂ or SpO₂/FiO₂) | PaO₂/FiO₂ >300 or SpO₂/FiO₂ >357 | PaO₂/FiO₂ 201–300 or SpO₂/FiO₂ 357–512 | PaO₂/FiO₂ ≤200 or SpO₂/FiO₂ ≤214 |
| Patient Group | Prevalence (% of patients) |
28-Day Mortality (%) | Assigned Grade |
| Absence of OF | 68.3 | 4.4 | Absence of ACLF |
| Single, nonkidney OF without KD or BD | 9.9 | 6.3 | Absence of ACLF |
| Single KF | 6.7 | 18.6 | ACLF-1a |
| Single, nonkidney OF with KD or BD | 4.2 | 27.8 | ACLF-1b |
| Two OFs | 7.5 | 32.0 | ACLF-2 |
| Three OFs | 1.9 | 68.0 | ACLF-3 |
| Four to six OFs | 1.4 | 88.9 | ACLF-3 |
Baveno VII stages of liver cirrhosis including portal hypertension
The international Baveno VII consensus brought about numerous innovations in the management of portal hypertension. The focus was on the non-invasive diagnosis of clinically significant portal hypertension defining five stages in advanced chronic liver disease:
Stage 1: Compensated liver cirrhosis without clinically significant portal hypertension
Stage 2: Compensated liver cirrhosis with clinically significant portal hypertension
Stage 3: First decompensation of liver cirrhosis
Stage 4: Further decompensation of liver cirrhosis
Stage 5: Recompensated liver cirrhosis
Definition of first decompensation and further decompensation (Baveno VII)
Compensated liver cirrhosis is progressing to decompensation at the time of first presence of one of the following complications: overt ascites, overt hepatic encephalopathy and variceal bleeding. At this time point it remains controversial whether minimal manifestation of the mentioned complication already define the development of hepatic decompensation. Of note, other complications such as onset of acute-on-chronic-liver-failure, development of hepatocellular carcinoma, superimposed liver injury, onset of infection and presence of jaundice do currently not define the progress to decompensated liver cirrhosis according to the Baveno consensus. The mortality increases significantly with onset of hepatic decompensation (de Franchis 2022).
Decompensated liver cirrhosis is divided into two stages: first decompensation and further decompensation. First decompensation is defined as first presence of overt ascites, overt hepatic encephalopathy or variceal bleeding. The prognosis worsens again with onset of further decompensation. Further decompensation is defined as either development of an additional second decompensating event or jaundice or the development of recurrent variceal bleeding, recurrent ascites, recurrent hepatic encephalopathy, spontaneous bacterial peritonitis or hepato-renal syndrome. Of note, in patients with variceal bleeding development of ascites, encephalopathy or jaundice at the time point of the bleeding is not considered as further decompensation. But development of either of these events after the bleeding defines the stage of further decompensation(de Franchis 2022).
Definition of recompensation
Despite the presence of a decompensating event in the past an improvement of liver disease is possible defined as the stage of recompensated liver disease. All of the following criteria have to be fulfilled: sufficient treatment of the primary aetio y of cirrhosis (e. g. alcohol abstinence, viral elimination of hepatitis c virus), for at least 12 months resolution of ascites (and no medication with diuretics), encephalopathy (no medication with lactulose and rifaximin) and absence of variceal bleeding and stable liver synthesis function (albumin, bilirubin, INR)(de Franchis 2022).
Clinical scores to determine the severity of liver cirrhosis
Several score systems are proposed to determine the severity of liver cirrhosis. The Child-Turcotte-Pugh score is one of the most commonly used scores in clinical practice and assigns the patient into one of three stages (A, B and C). It includes markers of liver synthesis function (albumin, INR), detoxification function (bilirubin, hepatic encepahalopathy) and portal hypertension (ascites).
Another commonly used score is the MELD (model for end stage liver disease). It is suggested to be more objective since it does not include subjective markers such as ascites and HE but only laboratory markers (bilirubin, creatinine,INR) (Durand 2005). The MELD score was developed to predict the mortality in patients with portal hypertension and implantation of a TIPS and application was expanded in all patients with liver cirrhosis (Kamath 2001).
Invasive tests
Liver biopsy
The term compensated advanced chronic liver disease (cACLD) has been defined by LSM to classify the progressive disease of severe fibrosis and cirrhosis in the Baveno VII criteria irrespective of histo ical features (de Franchis 2022). Although non-invasive testing and elastography are gradually taking over as mentioned below, liver biopsy remains a basic skill and necessity of the hepato ist’s diagnostic armamentarium to confirm diagnosis, assess stage and grade of the underlying chronic liver disease and to perform additional molecular analysis (Laleman 2023). The performance of a liver biopsy is the reference standard to assess the grade of liver fibrosis (European Association for the Study of the Liver 2021). Nevertheless, liver biopsy is also not always accurate as the quality of the specimen can differ and its interpretation can be quite complex requiring expertise in liver patho y. As with all invasive procedures complications such as bleeding especially in patients with impaired coagulation may occur (Davison 2020, Neuberger 2020). To improve the quality of pertcutaneous liver biopsy it is recommended to gain a sample length >15 mm with more than 10 portal tracts by a 16G needle and the assessment of a sample should be performed by an experienced patho ist (Neuberger 2020).
The liver biopsy is classified with the ISHAK score into seven categories (ranging from zero to six) according to the level of fibrosis in the sample. In the category 0 no evidence of fibrosis is present whereas in category 6 cirrhosis is probable or even diagnosed (Knodell 1981) (Table 3).
Table 3. ISHAK fibrosis stages| ISHAK Score | Fibrosis stage description |
| 0 | No fibrosis |
| 1 | Expansion of some portal areas, no septa |
| 2 | Expansion of most portal areas, rare septa |
| 3 | Portal fibrosis with occasional bridging septa |
| 4 | Portal fibrosis with frequent bridging septa |
| 5 | Incomplete cirrhosis (numerous septa but no true regenerative nodules) |
| 6 | Established cirrhosis with regenerative nodules |
The most common methods to perform a liver biopsy are a percutaneous or transjugular. The transjugular route should be preferred, if possible, in patients with a relevant coagulopathy (INR≥ 1.5) as the risk of bleeding is lower since the liver capsule is usually intact. Also, in patients with ascites a percutaneous biopsy is associated with a higher risk of bleeding and therefore a transjugular approach is better suited. Another advantage is the possibility of measurement the hepatic venous pressure gradient (HVPG) in the same procedure. However, the sample size is smaller and often more fragmented than in a percutaneous liver biopsy since a 18G or 19G needle is the standard needle in transjugular liver biopsy (Neuberger 2020).
In recent years, endoscopic ultrasound-guided liver biopsy EUS-LB has regained interest and has emerged as a well-tolerated, effective, and safe alternative to traditional liver tissue sampling (Laleman 2023). There are several benefits to EUS-LB in comparison to traditional approaches such as a lower perceived apprehension for the patient (given the use of sedation, lower post-procedural discomfort and shorter recovery), and the ability to target widely separated areas and even perform bilobar tissue sampling minimising as such sampling error and capturing inhomogeneous disease activity. Significant complications, bleeding and/or subcapsular hematoma, requiring emergency visit or hospitalisation occur about 1% of patients, similar to percutaneous approach (Baran 2021). Real-life data of percutaneous LBs showed that only 19% of cores are adequate, 56% suboptimal and 24% inadequate (Fryer 2013). A systematic review on EUS-LB, including 1326 patients showed a diagnostic yield of over 95% with an overall pooled mean tissue specimen length of 45.3 + 4.6mm containing 15.8 + 1.5 complete portal tracts (Pineda 2016). Currently, a 19G needle is favoured, and preferably the Franseen type (Laleman 2023).
Similarly to the transjugular approach, EUS-LB can be combined with direct EUS-guided portal pressure gradient (EUS-PPG) measurement as mentioned below. Therefore, endohepato y is not only conceptually and technically innovative but also highly practical for everyday use. It allows for a "one-stop clinic" approach, where patients can receive comprehensive endoscopic diagnostic and therapeutic procedures in a single outpatient visit.
Hepatic Venous Pressure Gradient (HVPG)
The gold standard to determine the presence of CSPH is the invasive performance of a hepatic venous pressure gradient (HVPG) measurement. This is an interventional technique that uses a transjugular venous access to place a catheter in a hepatic vein. The free hepatic venous pressure is then measured followed by measurement of the wedged hepatic venous pressure, which is created by inflating a balloon in the hepatic vein. The wedged hepatic venous pressure approximates the hepatic sinusoidal pressure and thus the portal venous pressure. The gradient between free and wedged hepatic venous pressure defines the HVPG. Clinically significant portal hypertension (CSPH) is defined as an HVPG ≥ 10 mmHg for most etio ies, especially viral- and alcohol-related cirrhosis (Table 4). CSPH defines a condition of high-risk for clinical portal hypertension related acute decompensation of compensated cirrhosis. At HVPG ≥ 10 mmHg patients are at risk of developing gastro-oesophageal varices, which then would indicate medical primary prophylaxis (de Franchis 2022, Villanueva 2019).
Table 4. Risk categories according to Hepatic Venous Pressure Gradient (HVPG)| HVPG | Risk category |
| <5 mmHg | normal |
| 5–9 mmHg | portal hypertension |
| ≥10 mmHg | clinical significant portal hypertension |
| >12 mmHg | high-risk for development of varices |
| >16 mmHg | high-risk variceal bleedings |
Endoscopic Ultrasound-guided Portal Pressure Gradient (EUS-PPG)
The gold standard for indirect measurement of portal vein pressure (HVPG) methodo ical limitations. It provides only an indirect measurement of portal pressure, relying on wedged hepatic vein pressure to reflect "free" sinusoidal perfusion and complete hepatic vein occlusion, thus unable to detect pre-sinusoidal and pre-hepatic portal hypertension. In clinical practice, this means HVPG measurement may underestimates the degree of portal hypertension in conditions like Primary Biliary Cholangitis (PBC) (Navasa 1987), Porto-Sinusoidal Vascular Disease (PSVD, previously Idiopathic Non-Cirrhotic Portal Hypertension) (De Gottardi 2019), or metabolic-associated fatty liver disease (MASLD) (Baffy 2022), as recently demonstrated for MASLD as one of the most common causes of chronic liver disease (Bassegoda 2022). By directly measuring portal and hepatic venous pressures, EUS-PPG avoids the risk of underestimated values in aforementioned clinical scenarios such as PBC, PSVD, and MASLD. For the large group of patients with liver cirrhosis (and sinusoidal portal hypertension), EUS-PPG can be used for personalised therapy management. In contrast to HVPG, EUS-PPG directly measures hepatic vein and portal pressures by transgastric puncture of these vessels under EUS guidance using a 25-G FNA needle.
While some authors describe that PPG values under sedation may be underestimated compared to awake measurements (Benmassaoud 2022, Reverter 2014), others showed that PPG values under Propofol sedation during EUS-PPG were even slightly higher compared to HVPG measurements without sedation (Martinez-Moreno 2024), suggesting inconclusive data on sedation's influence. Moreover, data on correlation of EUS-PPG with clinical outcome are still scarce.
Non-invasive tests
Liver stiffness measurement
Non-invasive strategies to determine PH are crucial to stratify patient care and to plan their clinical management. Since healthcare resources are limited, HVPG measurement, as a complex and invasive procedure, is only available in specialised centres and contains a periprocedural risk of bleeding and organ injury. Non-invasive tests (NIT) for CSPH are needed to guide patients’ management from a clinicians point-of-view, being useful in ruling out CSPH and therewith avoiding unnecessary examinations. On the other hand, they can rule in CSPH and can identify patients requiring further examinations or referral to a hepato ist (Brol 2023).
Liver fibrosis is the main mechanistic driver of portal hypertension. Portal hypertension is further aggravated by splanchnic blood flow and congestion. For a long time, histo ical analysis of liver biopsy was the most common tool to quantify liver fibrosis in patients with chronic liver disease, while HVPG was the gold standard for the diagnosis of CSPH. However, liver fibrosis and portal hypertension are both reflected in an increase in stiffness of the liver tissue due to congestion and fibrosis itself (Brol 2023).
Over the past few decades, non-invasive liver stiffness measurement (LSM) using transient elastography (TE) has emerged as a more widely spread method, nearly replacing liver biopsy for grading fibrosis in selected etio ies such as viral hepatitis and alcohol-related liver disease. Research has shown that TE correlates well with hepatic venous pressure gradient (HVPG), making it a useful tool for assessing high or low probability of the presence of clinically significant portal hypertension (CSPH). Today, TE is becoming more accessible and more frequently used to evaluate liver stiffness. However, LSM is not only determined by liver fibrosis but can be affected by different factors. As such recent food intake, cardiac congestion and hepatic inflammation can all increase LSM values, which needs to be taken into account when interpreting such LSM (Friedrich-Rust 2008). Successful measurements are validated using the following criteria: 1) number of valid shots ≥ 10; 2) ratio of valid shots to the total number of shots ≥ 60%; and 3) interquartile range (IQR, reflecting the variability of measurements) less than 30% of the median liver stiffness measurement (LSM) value (IQR/LSM ≤30%) (Ferraioli 2015).
Rule of five of liver stiffness measurement and platelet count
Both the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Disease (AASLD) recommend a threshold of >25 kPa, regardless of platelet count, to define high probability for the presence of CSPH in patients with virus-, alcohol-related and non-obese MASH-related etio y of liver disease. According to the Baveno VII consensus, when liver stiffness measurement values are between 15 and 25 kPa, platelet count should be considered for confirming high likelihood for the presence of CSPH in chronic liver disease.
CSPH is not likely present in patients with a liver stiffness measurement ≤15 kPa and a platelet count ≥150 x 109/L. Moreover, in the cACLD patients with liver stiffness measurement between 20–25 kPa, 15–20 kPa respectively and a platelet count ≤150 x 109/L, ≤110 x 109/L, respectively the probability for CSPH is increased (60 % risk). Further validation for use of the model in the aetio y of MASH is needed (Baveno VII) (Figure 1).
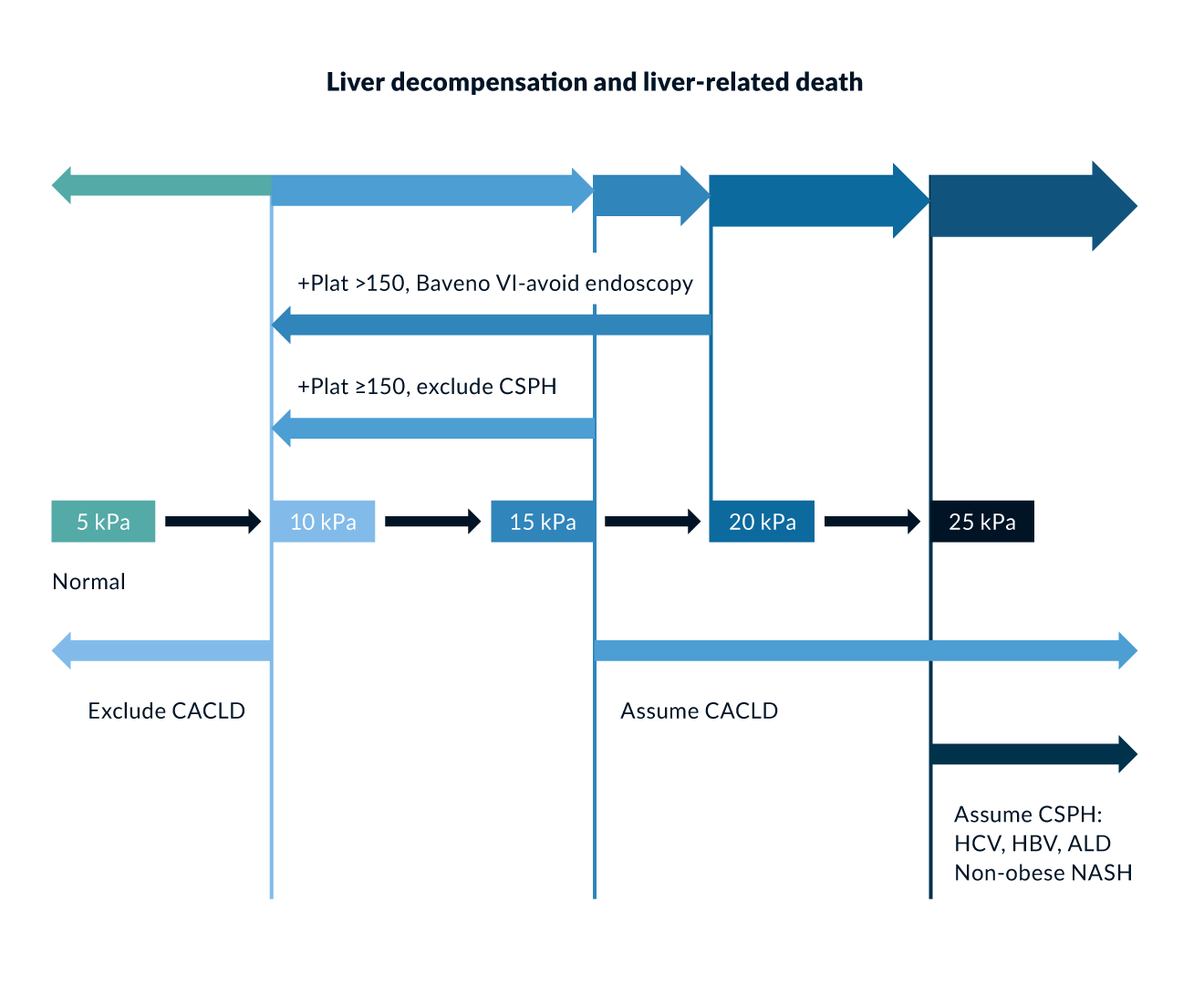 Figure 1. Algorithm for the noninvasive determination of cACLD and CSPH; from: Baveno VII – de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959-974
Figure 1. Algorithm for the noninvasive determination of cACLD and CSPH; from: Baveno VII – de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension. J Hepatol. 2022;76(4):959-974
Shear wave elastography (SWE), like transient elastography (TE), is used as an alternative technique to assess liver fibrosis. However, the comparability of studies is often challenged by the use of different manufacturers and varying elastography techniques, such as two-dimensional SWE (2D-SWE) or point SWE, depending on the device used. SWE is popular because it can be performed frequently and easily with standard ultrasound machines (Brol 2023).
One significant advantage of SWE over TE is its ability to be performed independently of the presence of ascites. Several earlier studies indicated that SWE was more effective in diagnosing clinically significant portal hypertension (CSPH) in patients with ascites and does not seem inferior to TE (Elkrief 2015, Leung 2013). However, the cut-off values for diagnosing CSPH depend on the specific ultrasound device used and may vary based on the underlying disease etio y.
More recent developments use LSM as a biomarker, that can be incorporated into predictive algorithms alongside other biomarkers. For example, the M10LS20 algorithm incorporates MELD and LSM identifying high-risk of death in patients with MELD >10 points and LSM > 20kPa (Trebicka 2022).
Spleen stiffness measurement
During portal hypertension (PH), the pressure in the splenic vein increases. This congestion of blood in the spleen causes it to enlarge, making spleen stiffness a reliable indicator for clinically significant portal hypertension (CSPH). In healthy adults, the average spleen stiffness measurement (SSM) is around 18 kPa, but it significantly increases in patients with CSPH (Kani 2022).
A meta-analysis of nine studies found that SSM measured by ultrasound-based elastography demonstrated a strong correlation with hepatic venous pressure gradient (HVPG), effectively detecting clinically significant portal hypertension (CSPH) (Song 2018). According to the new Baveno VII consensus statement, spleen stiffness (SSM) can be used to rule out (SSM <21 kPa) and rule in (>50kPa) CSPH. Moreover, for patients, who cannot take non-selective beta-blockers (due to contraindications or intolerance) and who would typically require an endoscopy based on the Baveno VI criteria (LSM by TE ≥20 kPa or platelet count ≤150 x 109/L), an SSM ≤40 kPa by TE can be used to identify those with a low risk of high-risk varices, allowing endoscopy to be avoided (Dajti 2023). The main advantage of including SSM in the diagnostic of CSPH is the reduction of the diagnostic grey zone. This leads to further reduction in unnecessary endoscopy to rule out varices. Hemato ical disorders, such as acute myeloid leukaemia and bone marrow fibrosis, have been identified as factors that can increase spleen stiffness.
Blood-based tests
Noninvasive assessment of CSPH using laboratory tests is convenient as it eliminates the need for technical expertise or specific devices. This convenience has led to numerous efforts to develop predictive algorithms for CSPH.
FIB-4 score
The Fibrosis-4 index (FIB-4) is a serum-based noninvasive score used to predict liver fibrosis, based on age, alanine aminotransferase (ALT) level, aspartate aminotransferase (AST) level, and platelet count. Initially developed for predicting liver fibrosis in patients coinfected with HIV and HCV, its predictive value has been validated for liver fibrosis of various other etio ies. The advantages of the score are the broad availability, good accuracy for advanced liver fibrosis and rather low costs (European Association for the Study of the Liver 2021). Recent retrospective studies have shown that FIB-4 can be used with adjusted thresholds to predict clinically significant portal hypertension (CSPH). However, for the use in primary care not many validation studies were performed yet and misclassification is possible (European Association for the Study of the Liver 2021, Ginès 2022). The Fib-4 score is best used to exclude the presence of fibrosis than to diagnose the presence of fibrosis. However, especially in patients older than 65 years false positive results are common. Additionally, a significant number of patients have a FIB-4 score within a transitional range with no clear recommendation being defined (EASL guideline noninvasive tests 2021). A FIB-4 value below 1.30 is considered as low risk for advanced fibrosis; a value over 2.67 is considered as high risk for advanced fibrosis; and FIB-4 values between 1.30 and 2.67 are considered as intermediate risk of advanced fibrosis. (European Association for the Study of the Liver 2021)
MAFLD fibrosis score
To calculate the NAFLD fibrosis score (MFS) (former NAFLD fibrosis score) age, BMI, diabetes, aminotransferases, platelets and albumin were taken into consideration (European Association for the Study of the Liver 2021). In patients with diabetes and obesity the performance of NFS seems to be impaired. Therefore, in these patients the FIB-4 score might be preferred (European Association for the Study of the Liver 2021). Similar to the FIB-4 score the MFS can show false positive results in patients older than 65 years.
However, in MASH the performance of a liver biopsy remains the standard procedure to determine the diagnosis as all noninvasive tests are not reaching an acceptable level of accuracy(European Association for the Study of the Liver 2021).
Conclusion
The grading and staging of liver diseases are crucial for assessing disease severity, guiding treatment decisions, and predicting patient outcomes. Cirrhosis and portal hypertension represent advanced stages of liver disease, with clinically significant portal hypertension (CSPH) being a major determinant of morbidity and mortality.
Non-invasive methods such as liver stiffness measurement (LSM) and spleen stiffness measurement (SSM) have largely replaced liver biopsy for fibrosis staging, aligning with the Baveno VII recommendations. Blood-based tests like FIB-4 and the MAFLD fibrosis score offer additional tools for fibrosis assessment, though their predictive accuracy varies by patient demographics and disease etio y.
Despite advancements in non-invasive diagnostics, hepatic venous pressure gradient (HVPG) measurement remains the gold standard for CSPH assessment, particularly for conditions like viral and alcohol-related cirrhosis. EUS-guided techniques, including endoscopic ultrasound-guided liver biopsy (EUS-LB) and endoscopic ultrasound-guided portal pressure gradient (EUS-PPG) measurement, are gaining attention as less invasive alternatives that directly measure portal pressures and allow for more precise, comprehensive tissue sampling. These EUS-guided approaches overcome technical limitations of HVPG by providing more accurate portal pressure measurements, especially in conditions with pre-sinusoidal or pre-hepatic portal hypertension, and offer the benefit of real-time tissue acquisition under ultrasound guidance. However, its predictive value on clinical outcomes have to be determined before broad clinical use can be recommended.
Clinical classification systems, including the NAD, AD, ACLF classification or the Baveno VII staging as well as scoring systems such as Child-Pugh and MELD, provide structured frameworks for evaluating liver disease progression. The concept of recompensated cirrhosis highlights the potential for disease improvement with targeted treatment strategies.
Moving forward, a multimodal approach combining non-invasive tests, laboratory biomarkers, imaging techniques, and EUS-guided methods will be essential for optimising liver disease management while minimising procedural risks for patients. Further validation and standardisation of these non-invasive and EUS-guided methods will be key to their broader clinical implementation.
References
Arroyo V, Moreau R, Jalan R. Acute-on-Chronic Liver Failure. N Engl J Med. 2020;382(22):2137-2145.
Baffy G, Bosch J. Overlooked subclinical portal hypertension in non-cirrhotic NAFLD: Is it real and how to measure it?. J Hepatol. 2022;76(2):458-463.
Baran B, Kale S, Patil P, et al. Endoscopic ultrasound-guided parenchymal liver biopsy: a systematic review and meta-analysis. Surg Endosc. 2021;35(10):5546-5557.
Bassegoda O, Olivas P, Turco L, et al. Decompensation in Advanced Nonalcoholic Fatty Liver Disease May Occur at Lower Hepatic Venous Pressure Gradient Levels Than in Patients With Viral Disease. Clin Gastroenterol Hepatol. 2022;20(10):2276-2286.e6.
Benmassaoud A, Chen YI. Sedation during EUS-guided portal pressure gradient measurement: the elephant in the room. Gastrointest Endosc. 2022;96(4):690-691.
Brol MJ, Gödiker J, Uschner FE, Praktiknjo M, Trebicka J. Non-invasive Assessment of Clinically Significant Portal Hypertension. Current Hepatology Reports, 22(3), 206–215.
Dajti E, Ravaioli F, Zykus R, et al. Accuracy of spleen stiffness measurement for the diagnosis of clinically significant portal hypertension in patients with compensated advanced chronic liver disease: a systematic review and individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2023;8(9):816-828.
D'Amico G, Morabito A, D'Amico M, et al. Clinical states of cirrhosis and competing risks. J Hepatol. 2018;68(3):563-576.
Davison BA, Harrison SA, Cotter G, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73(6):1322-1332.
de Franchis R, Bosch J, Garcia-Tsao G, Reiberger T, Ripoll C; Baveno VII Faculty. Baveno VII - Renewing consensus in portal hypertension [published correction appears in J Hepatol. 2022 Jul;77(1):271. doi: 10.1016/j.jhep.2022.03.024.]. J Hepatol. 2022;76(4):959-974.
De Gottardi A, Rautou PE, Schouten J, et al. Porto-sinusoidal vascular disease: proposal and description of a novel entity. Lancet Gastroenterol Hepatol. 2019;4(5):399-411.
Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. J Hepatol. 2005;42 Suppl(1):S100-S107.
Elkrief L, Rautou PE, Ronot M, et al. Prospective comparison of spleen and liver stiffness by using shear-wave and transient elastography for detection of portal hypertension in cirrhosis. Radiology. 2015;275(2):589-598.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018;69(2):406-460.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines on acute-on-chronic liver failure. J Hepatol. 2023;79(2):461-491.
European Association for the Study of the Liver. Electronic address: easloffice@easloffice.eu; European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis - 2021 update. J Hepatol. 2021;75(3):659-689.
Ferraioli G, Filice C, Castera L, et al. WFUMB guidelines and recommendations for clinical use of ultrasound elastography: Part 3: liver. Ultrasound Med Biol. 2015;41(5):1161-1179.
Friedrich-Rust M, Ong MF, Martens S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960-974.
Fryer E, Wang LM, Verrill C, Fleming K. How often do our liver core biopsies reach current definitions of adequacy?. J Clin Pathol. 2013;66(12):1087-1089.
Ginès P, Castera L, Lammert F, et al. Population screening for liver fibrosis: Toward early diagnosis and intervention for chronic liver diseases. Hepatology. 2022;75(1):219-228.
Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33(2):464-470.
Kani HT, Keklikkıran Ç, Ergenç İ, Yılmaz Y. Evaluation of spleen stiffness in healthy population: A vibration-controlled transient elastography study. Journal of Health Sciences and Medicine. 2022;5(2), Article 2.
Knodell RG, Ishak KG, Black WC, et al. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981;1(5):431-435.
Laleman W, Mertens J, Vanderschueren E, Praktiknjo M, Trebicka J. Advances in Endohepatology. Am J Gastroenterol. 2023;118(10):1756-1767.
Leung VY, Shen J, Wong VW, et al. Quantitative elastography of liver fibrosis and spleen stiffness in chronic hepatitis B carriers: comparison of shear-wave elastography and transient elastography with liver biopsy correlation. Radiology. 2013;269(3):910-918.
Martinez-Moreno B, Martínez Martínez J, Herrera I, et al. Correlation of endoscopic ultrasound-guided portal pressure gradient measurements with hepatic venous pressure gradient: a prospective study. Endoscopy. 2025;57(1):62-67.
Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144(7):1426-1437.e14379.
Navasa M, Parés A, Bruguera M, Caballería J, Bosch J, Rodés J. Portal hypertension in primary biliary cirrhosis. Relationship with histological features. J Hepatol. 1987;5(3):292-298.
Neuberger J, Patel J, Caldwell H, et al. Guidelines on the use of liver biopsy in clinical practice from the British Society of Gastroenterology, the Royal College of Radiologists and the Royal College of Pathology. Gut. 2020;69(8):1382-1403.
Pineda JJ, Diehl DL, Miao CL, et al. EUS-guided liver biopsy provides diagnostic samples comparable with those via the percutaneous or transjugular route. Gastrointest Endosc. 2016;83(2):360-365.
Reverter E, Blasi A, Abraldes JG, et al. Impact of deep sedation on the accuracy of hepatic and portal venous pressure measurements in patients with cirrhosis. Liver Int. 2014;34(1):16-25.
Schulz MS, Angeli P, Trebicka J. Acute and non-acute decompensation of liver cirrhosis (47/130). Liver Int. 2025;45(3):e15861.
Tonon M, D'Ambrosio R, Calvino V, et al. A new clinical and prognostic characterization of the patterns of decompensation of cirrhosis. J Hepatol. 2024;80(4):603-609.
Trebicka J, Fernandez J, Papp M, et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J Hepatol. 2021;74(5):1097-1108.
Trebicka J, Fernandez J, Papp M, et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J Hepatol. 2020;73(4):842-854.
Trebicka J, Gu W, de Ledinghen V, et al. Two-dimensional shear wave elastography predicts survival in advanced chronic liver disease. Gut. 2022;71(2):402-414.
Villanueva C, Albillos A, Genescà J, et al. β blockers to prevent decompensation of cirrhosis in patients with clinically significant portal hypertension (PREDESCI): a randomised, double-blind, placebo-controlled, multicentre trial [published correction appears in Lancet. 2019 Jun 22;393(10190):2492.
