4. Hepatitis D – diagnosis and treatment
Lisa Sandmann, Heiner Wedemeyer, Markus Cornberg
Introduction
The hepatitis delta virus (HDV) is a defective RNA virus which requires the hepatitis B virus (HBV) surface antigen (HBsAg) for generation of infectious virus particles and transmission, while the full extent of the HBV helper function is unexplored (Rizzetto 1983, Taylor 2012). Hence, HDV occurs only in HBsAg positive individuals either as acute coinfection or as superinfection in patients with chronic HBV (Wedemeyer 2010b) (Figure 1). Several studies have shown that chronic HDV infection leads to more severe liver disease than chronic HBV monoinfection, with an accelerated course of fibrosis progression, an increased risk of hepatocellular carcinoma and early decompensation in the setting of established cirrhosis (Beguelin 2017b, Hughes 2011, Manesis 2013). Currently, two treatment options are available and recommended by guidelines (EASL 2023, Sandmann 2023a). The entry-inhibitor bulevirtide has been approved by EMA. Results from phase 2 and 3 studies were published (Wedemeyer 2023a, Wedemeyer 2023c) and confirmed in real-world cohort analyses (Degasperi 2022a, Dietz-Fricke 2023) In the phase 3 study, on-treatment rates of combined response were 45% and 55% at 48 or 96 weeks of treatment, respectively (Wedemeyer 2024, Wedemeyer 2023a). Currently, treatment is recommended indefinitely for as long as the patient is benefitting (EMA 2024a). This is in contrast to treatment with pegylated interferon alfa (PEG-IFN) in which a defined treatment duration of 48 weeks is recommended (EASL 2023, Sandmann 2023a). About one quarter of patients showed prolonged virological off-treatment response but long-term HDV RNA relapses may occur (Heidrich 2014). HBsAg clearance should be the preferred endpoint of interferon-based therapies of HDV, but this is rarely achieved. Yet, suppression of HDV RNA in the presence of HBsAg has been associated with improved clinical outcomes (Farci 2004, Wranke 2017, Yurdaydin 2018a). Additional treatment options are currently in clinical development.
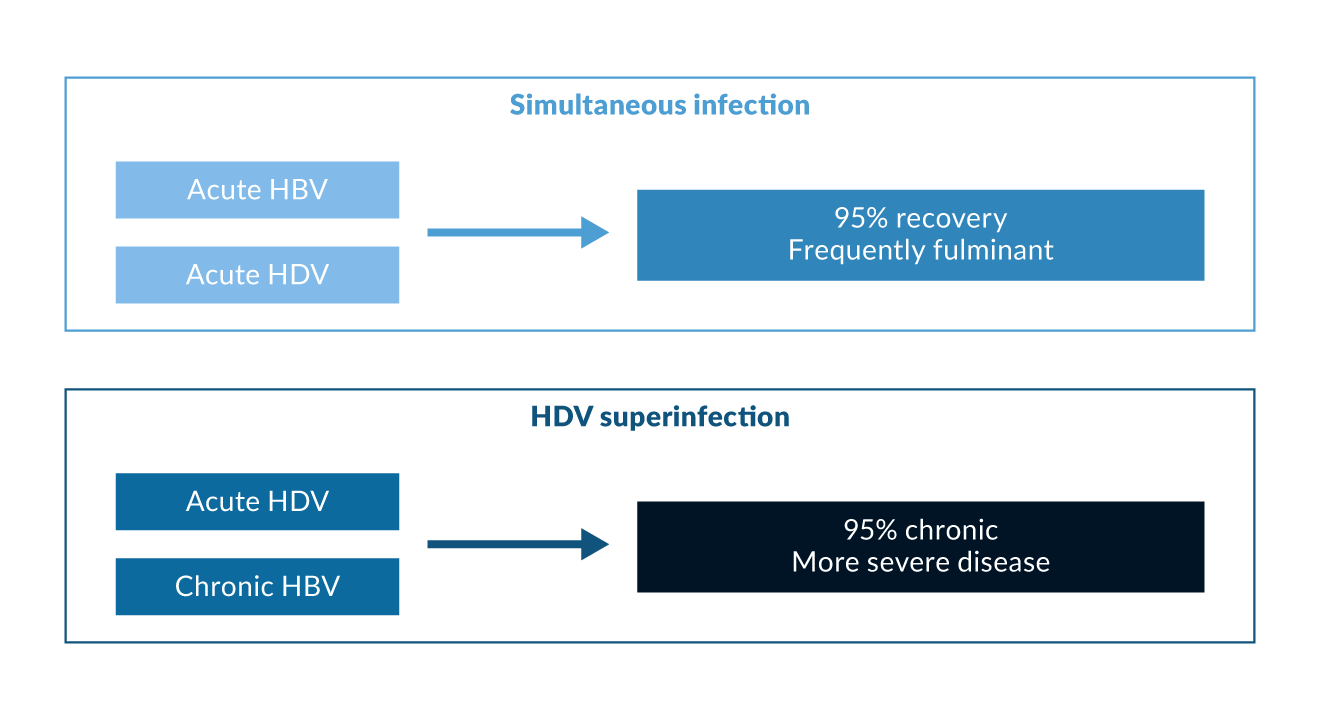 Figure 1. Courses of hepatitis delta
Figure 1. Courses of hepatitis delta
Virology of HDV
The hepatitis D virion is approximately 36 nm in size, containing HDV RNA and delta antigen. HDV RNA is single-stranded, highly base-paired, circular and by far the smallest known genome of any animal virus, containing close to 1700 nucleotides (Sureau 2016, Taylor 2012). It is coated with the envelope protein derived from the pre-S and S antigens of HBV. Other enveloped viruses including HCV and VSV can also propagate HDV infection, both in vitro as well in humanized mice (Perez-Vargas 2019). Still, it is currently unclear if viruses distinct from HBV induce dissemination of HDV also in patients. The HDV RNA has six open reading frames (ORFs), three on the genomic and three on the antigenomic strand. One ORF codes for the hepatitis delta antigen (HDAg), while the other ORFs do not appear to be actively transcribed. Two HDAgs exist: the small HDAg (24 kD) is 155 amino acids long and the large HDAg (27 kD) is 214 amino acids long. A single nucleotide change (A-G) in the small HDAg sequence leads to the synthesis of the large HDAg. The small HDAg accelerates genome synthesis, while the large HDAg that inhibits HDV RNA synthesis is necessary for virion morphogenesis (Taylor 2012). Replication of HDV RNA occurs through a ‘double rolling circle’ model in which the genomic strand is replicated by a host RNA polymerase to yield a multimeric linear structure that is then autocatalytically cleaved to linear monomers and ligated into the circular HDV RNA viral progeny (Sureau 2016). Recent work showed that the host RNA polymerase II-is coactivated by S-HDAg using a histone mimicry strategy (Abeywickrama-Samarakoon 2020).
Genetic analysis has revealed the presence of at least eight HDV genotypes (Le Gal 2017) (Figure 2). Genotype 1 is the most frequently seen and is distributed throughout the world, especially in Europe, the Middle East, North America and North Africa. Genotype 2 is seen in East Asia and the Yakutia region of Russia, and genotype 3 is present exclusively in the northern part of South America, especially in the Amazon basin. Genotype 4 is seen in Taiwan and Japan, while genotypes 5-8 are found in Africa (Deny 2006). HDV genotype 1 is associated with both severe and mild disease whereas genotype 2 causes a milder disease over a long-term course (Su 2006). HDV genotype 5 may also take a milder course and a better response to PEG-IFN treatment compared to genotype 1 (Spaan 2020).
HDV quasispecies evolution declines over time during HDV infection even though a continuous adaptation of HDV occurs indicating ongoing immune pressure in chronic HDV (Homs 2016).
HBV genotypes may also contribute to distinct clinical courses of HDV. There is no evidence that specific HDV genotypes may infect patients with one specific HBV genotype exclusively. However, data indicate that distinct HDV mutations may facilitate association of certain HDV genotypes with different HBV genotypes (Kay 2014). The global distribution of HBV and HDV genotypes is shown in Table 1.
Table 1. HBV and HDV genotypes| Region | HDV genotype | HBV genotype |
| Europe | 1 | D/A |
| Brazil | 1/3 | F/A/D |
| China, Taiwan, Japan | 1/2/4 | B/C |
| Turkey, Iran, Pakistan, India | 1 | D |
| Western Pacific | 1/2 | B/C/D |
| Africa | 1, 5–8 | D/A/E |
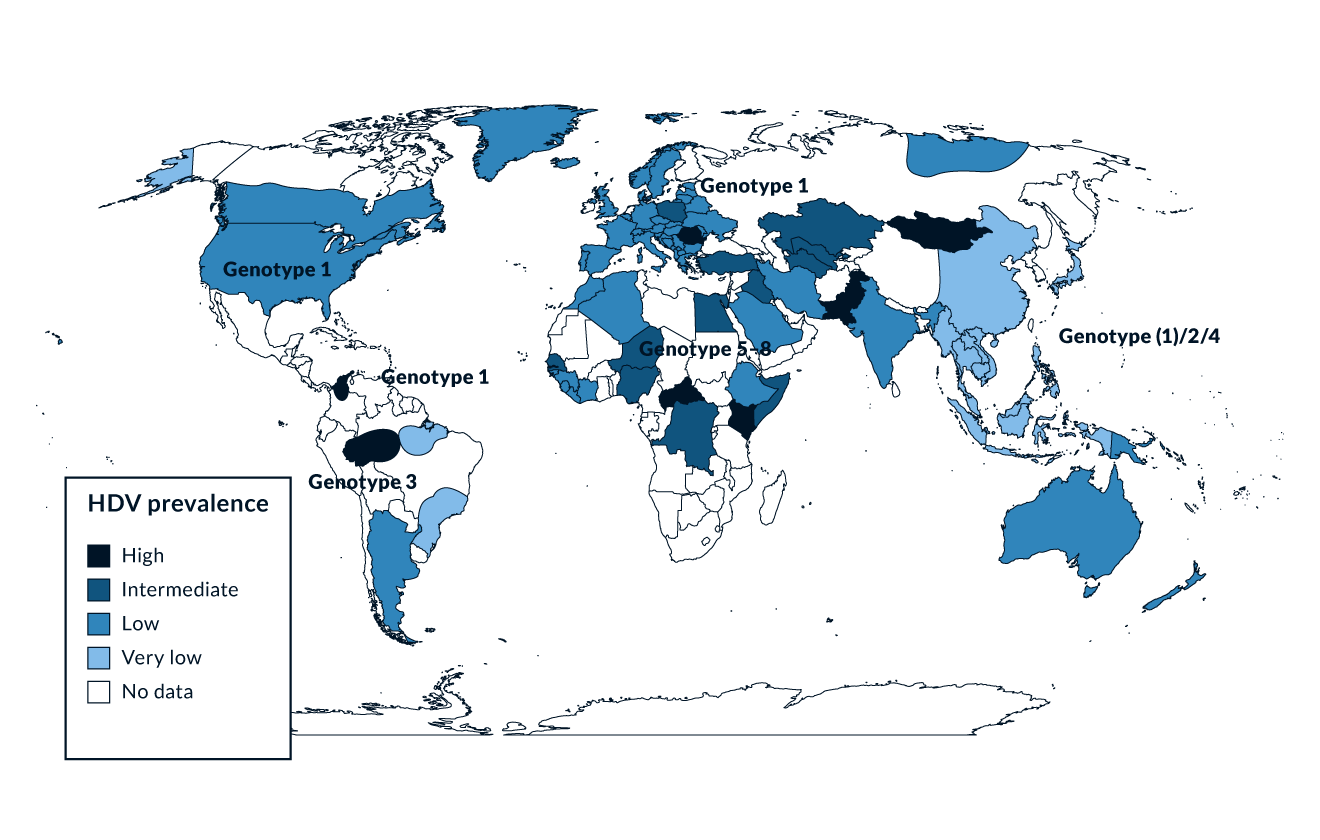 Figure 2. HDV prevalence
Figure 2. HDV prevalence
Epidemiology of HDV
Being linked to HBV, HDV is spread in the same way as HBV, mainly through parenteral exposure (Niro 1999). Worldwide, 217 to 316 million people are chronically infected with HBV (Polaris Observatory 2023) and 9–19 million of those are estimated to be anti-HDV positive (Stockdale 2020). However, conflicting data on the prevalence of HDV exists (Wedemeyer 2020) which might be partially due to different testing strategies that are currently present. Risk-based testing is recommended by the AASLD guideline (Terrault 2018) while anti-HDV testing for all HBsAg positive samples is recommended by EASL (EASL 2023). In high-income countries, high anti-HDV prevalence is found in people who inject drugs (PWID) who are HBsAg positive, both in Europe (Erhardt 2010, Gaeta 2000, Heidrich 2009) and North America (Kucirka 2010). Historically, HDV was endemic in Southern Europe. Several studies performed in the 1980s and 1990s showed a prevalence of anti-HDV of more than 20% among HBsAg positive individuals. As a result of the implementation of HBV vaccination programs, the incidence of HDV infections significantly decreased in Southern Europe in the 1990s (Degertekin 2008, Gaeta 2000). Countries with a particularly high prevalence of HDV are Mongolia with up to one third of chronic hepatitis cases being caused by HDV (Tsatsralt-Od 2005), Romania (Gheorghe 2015), some Central Asian countries like Uzbekistan (Khodjaeva 2019) and Pakistan (Abbas 2012), northwestern states of Brazil (Braga 2014, Kay 2014), distinct regions in Africa (Andernach 2014), and some Polynesian islands (Han 2014) (Figure 2). Of note, prevalence rates of HBV and HDV are not linked - for example, HDV infections have been considered to be rare in most parts of mainland China despite very high frequencies of HBV. However, some studies revealed an HDV prevalence of up to 6.5%, suggesting that HDV may be more frequent in China than previously thought (Liao 2014). In Taiwan, a country with a well-established national HBV vaccination program, the epidemiology of HDV changed over the last 20 years with PWID and HIV positive persons being particular risk groups and representing a main reservoir for HDV infection (Hung 2014, Lee 2015, Lin 2015). Thus, even though HDV is a major problem in distinct regions and specific cohorts, HDV is overall a rare disease and has therefore been granted orphan designation both by the FDA and by the European Commission.
One problem is that many HBsAg positive patients are not tested for HDV. The HDV testing rate was low in four hospitals in London where people with HDV frequently had severe disease and patients were of very diverse ethnicity (El Bouzidi 2015). In the United States Veterans Affairs medical system, only 8.5% of more than 25,000 HBsAg positive patients were tested for HDV. Of those, 3.4% had evidence for HDV and HDV was associated with a 2.9-fold higher HCC incidence and a higher risk of all-cause mortality (Kushner 2015). Recent studies evaluated the effects of reflex testing in HBsAg positive individuals (Palom 2022). In doing so, the absolute number of HDV diagnoses quintrupled compared to the era without reflex testing. A current modelling analysis from the Polaris Observatory recommends double reflex testing (anti-HDV testing for all HBsAg positive individuals followed by HDV RNA testing in anti-HDV positive samples) for the correct estimation of the worldwide HDV prevalence (Razavi 2023).
Pathogenesis of HDV
Knowledge about the pathogenesis of HDV infection is limited. Clinical observations have provided examples of mostly an immune-mediated process in HDV (Grabowski 2010). However, patterns suggesting a cytopathic viral disease have occasionally been observed. A typical example of this were outbreaks of severe hepatitis in the northern part of South America (Nakano 2001). These mostly fulminant hepatitis cases were induced by genotype 3 HDV. In HDV, liver histology is not different from a patient with HBV or HCV with accompanying necroinflammatory lesions. Importantly, HDV viremia is not directly associated with the stage of liver disease in HDV genotype 1 infection (Zachou 2010) while in HDV genotype 3 infection higher viral loads were observed in patients with cirrhosis (Braga 2014). In both humanized chimeric mice as well as mice expressing the human HBV receptor (sodium taurocholate co-transporting polypeptide (NTCP)) HDV infection provoked a marked and broad induction of interferon stimulated genes and cytokines which was more pronounced than in HBV monoinfection (Giersch 2015, He 2015) which may directly contribute to the more severe inflammation in patients with HDV. Another study showed that modification of three amino acids in mouse NTCP (H84R, T86K, and S87N) rendered mice susceptible to HDV (He 2016). In this respect it is important to note that distinct polymorphisms in the IL28B gene may be associated with HBsAg persistence also in HDV coinfected patients (Karatayli 2015).
Cellular immune responses against the HDV have been described (Hoblos 2023, Huang 2004, Nisini 1997) suggesting that the quantity and quality of T cell responses may be associated with some control of the infection. HDV-specific IFN gamma and IL-2 responses are more frequent in patients with low HDV viraemia (Grabowski 2011). However, HDV-specific T cell responses are very weak and exhausted in chronic infection. In vitro, the third signal cytokine IL-12 was able to restore the function of HDV-specific CD4+ and CD8+ T cells (Schirdewahn 2017). In addition to immune exhaustion, T cell failure may also be caused by T cell escape variants (Karimzadeh 2018, Karimzadeh 2019, Kefalakes 2019). However, T cell responses in the liver may also lead to immune pathogenesis. One study investigated innate and adaptive immune responses localized in the liver and showed that also liver-resident CD8+ T cells, and in particular antigen-nonspecific T cells, contribute to liver disease pathogenesis (Kefalakes 2021). NK cells from patients with HDV have recently been investigated in more detail (Lunemann 2014). Overall, NK cell frequencies increased but the cells were less activated and functionally impaired. HDV infection also did not alter NK cell differentiation, and the activity of liver disease reflected alterations in NK cell surface receptor expression. NK cell frequency may also be associated with early virological response to PEG-IFN therapy although NK cells are severely functionally impaired during antiviral therapy (Lunemann 2015). Finally, mucosa-associated invariant T (MAIT) cells, which are innate-like T cells highly enriched in the human liver, are activated, functionally impaired and severely depleted in patients with chronic hepatitis D (Dias 2019). This loss of MAIT cells was associated with severity of liver disease. Collectively, this information suggests that HDV is mainly an immune-mediated disease, at least in HDV genotype 1 infection. Ideally, antiviral therapies should therefore also aim to enhance anti-HDV immunity to confer long-term control of the infection.
Coinfections with multiple hepatitis viruses are associated with diverse patterns of reciprocal inhibition of viral replication (Raimondo 2006, Wedemeyer 2010a). HDV has frequently been shown to suppress HBV replication (Calle Serrano 2012). Between 70% and 90% of HDV patients are HBeAg negative with low levels of HBV DNA. Humanized HBsAg positive mice that become superinfected with HDV also show a decrease in HBV replication (Lutgehetmann 2012). A molecular explanation for the suppression of HBV replication by HDV has been suggested via the HDV proteins p24 and p27 repressing HBV enhancers (Williams 2009). In addition, induction of a type-I interferon response by HDV may contribute to HBV repression. This hypothesis is supported by the induction of interferon stimulated genes in HBV cells which were superinfected with HDV which led to a decrease of HBV replication markers (Alfaiate 2016). Viral dominance may change over time and about half of the hepatitis delta patients showed significant HBV replication in one study (Schaper 2010).
HDV may also play a direct role in the development of hepatocellular carcinoma by altering DNA methylation events (Benegiamo 2013). Recent systematic reviews and meta-analyses noted a significantly higher risk of HCC development in HDV compared to HBV monoinfection (Alfaiate 2020, Kamal 2021). If this higher risk is due to earlier development of liver cirrhosis or a consequence of direct oncogenic effects of HDV is a matter of debate.
Clinical course of HDV
Acute HBV/HDV coinfection
Acute HBV/HDV coinfection in adults leads to recovery in more than 90% of cases but frequently causes severe acute hepatitis with a high risk for developing a fulminant course (Rizzetto 2009). In contrast, HDV is cleared spontaneously only in a minority of patients with HDV superinfection of chronic HBsAg carriers (Figure 1). The observation that the histopathology of simultaneous HBV and HDV infection is more severe than in infection with HBV alone has also been documented in experiments with chimpanzees (Dienes 1990). Several outbreaks of very severe courses of acute HDV have been described in different regions of the world (Casey 1996, Flodgren 2000, Tsatsralt-Od 2006). Fortunately, acute HDV has become infrequent over the last two decades in high-income countries due to the introduction of vaccination programs.
Chronic HDV infection
Several early studies showed that chronic HDV leads to more severe liver disease compared to chronic HBV monoinfection, with an accelerated course of fibrosis progression, and early decompensation in the presence of cirrhosis (Asselah 2023, Wranke 2023, Wranke 2024). Long- term follow-up data from Italy, Spain, Greece and Germany confirmed the particularly severe course of HDV (Buti 2011, Calle Serrano 2014, Manesis 2013, Niro 2010, Romeo 2009). Characteristics of patients with HDV genotype 3 infection were reported in more detail confirming the severity of liver disease also for this specific HDV genotype (Braga 2014). HDV infection has been associated with a particular high risk of developing liver cirrhosis in people who are living with HIV (Calle Serrano 2012, Fernandez-Montero 2014). In one cross-sectional study from Spain, 66% of people coinfected with HIV/HBV/HCV/HDV presented with liver cirrhosis compared to only 6% of people coinfected with only HBV/HCV/HIV (Castellares 2008) and this translated to higher rates of liver decompensation and death (Fernandez-Montero 2014). Similarly, HDV was associated with poorer survival in HIV positive people in Taiwan (Lee 2015, Sheng 2007) and in the Swiss HIV cohort study (Beguelin 2017b). The Swiss study showed a prevalence of HDV of 15.4% and showed a 2.3- fold increased risk of overall death for those coinfected with HIV/HDV. Furthermore, a six-fold increased risk of HCC was calculated for HIV/HBV/HDV triple infected patients (Kamal 2021). Recent data from Sweden showed that HDV infection was associated with a 3.8-fold higher risk for liver related outcomes (Kamal 2020).
An easy-to-apply clinical score, the baseline-event anticipation (BEA) score, has been suggested to predict the risk of developing liver-related morbidity and mortality (Calle Serrano 2014). Factors associated with a poor long-term outcome included age above 40, male sex, low platelet counts, high bilirubin and INR values and southeast Mediterranean origin. The BEA score was validated in two independent European cohorts. However, the cohort size was limited (n=77 and 62, respectively), so the use of the score has not yet become widespread.
Diagnosis of HDV
Current EASL guidelines recommend that everyone who is HBsAg positive should be tested for anti-HDV antibodies at least once (Figure 3). Testing should be repeated whenever clinically indicated, e.g. in case of elevated liver enzymes or decompensation of chronic liver disease (EASL 2023, Sandmann 2023a).
In case of positive anti-HDV, HDV RNA testing should be performed with a standardized and sensitive nucleic acid test. It is important to note that the sensitivity of available HDV RNA assays varies (Le Gal 2016) and also the extraction method has an influence on the viral load quantification (Bremer 2019). This has to be taken into account when comparing results from different laboratories (Sandmann 2024a, Wedemeyer 2023b). In case of detectable HDV RNA subsequent evaluation of grading and staging of liver disease, surveillance for hepatocellular carcinoma and consideration of antiviral treatment is indicated (EASL 2023, Sandmann 2023a). So far, there is no consistent evidence that HDV RNA levels are strongly correlated with histological markers of liver disease (Zachou 2010) even though high HDV RNA levels may be predictive of developing cirrhosis and HCC in the long term (Romeo 2014). HDV genotyping may help to stratify patients, e.g. identify patients with a higher or lower risk of developing end-stage liver disease (Su 2006). In high-income countries, almost all patients are infected with HDV genotype 1, thus genotyping may be considered mainly in immigrants or populations with mixed genotype prevalence. However, genotyping is no prerequisite for antiviral treatment and can be omitted based on current treatment guidelines (Sandmann 2023a). As HDV occurs only in the context of HBV coinfection, a work-up of HBV infection including HBV DNA quantification and HBeAg/anti-HBe determination is warranted. Between 10% and 20% of HDV patients are HBeAg positive. Of note, HBV DNA can be suppressed even in HBeAg positive hepatitis (Heidrich 2012) suggesting that the inhibitory effect of HDV on HBV is independent from the phase of HBV infection. The long-term clinical outcome of anti-HDV positive patients did not differ between HBeAg positive and HBeAg negative individuals in one study from Germany (Heidrich 2012). Most HDV patients in Europe are infected with HBV genotype D but infection with genotype A can also occur (Soriano 2011). Because of the similar risk profiles of the patients, tests for HIV and HCV are also mandatory.
Quantitative HBsAg levels may be helpful for therapeutic management in certain situations (Sandmann 2023a). During treatment with PEG-IFN, a strong HBsAg decline may be a reason to extend the treatment duration from 48 to 96 weeks. During bulevirtide (BLV) monotherapy no effect on HBsAg has been observed so far. Therefore, quantitative determination is not mandatory during BLV treatment. Staging of liver disease is of particular importance in HDV as treatment options are still limited. Various non-invasive serum markers have been developed to predict liver fibrosis and cirrhosis in HCV, HBV and MASLD. However, scores such as APRI, FIB-4 or AST/ALT ratio have to be used with caution in HDV infection (Da 2020, Lutterkort 2017, Sandmann 2024b, Takyar 2017). Novel scores specifically developed for HDV have been proposed. One score is based on serum cholinesterase, gamma glutamyl transferase, albumin and age and has been validated in European patients (Lutterkort 2017). Transient elastography has been shown to be useful to exclude liver cirrhosis (<15 kPa) and advanced fibrosis (<10 kPa) in HDV patients (Sandmann 2024b).
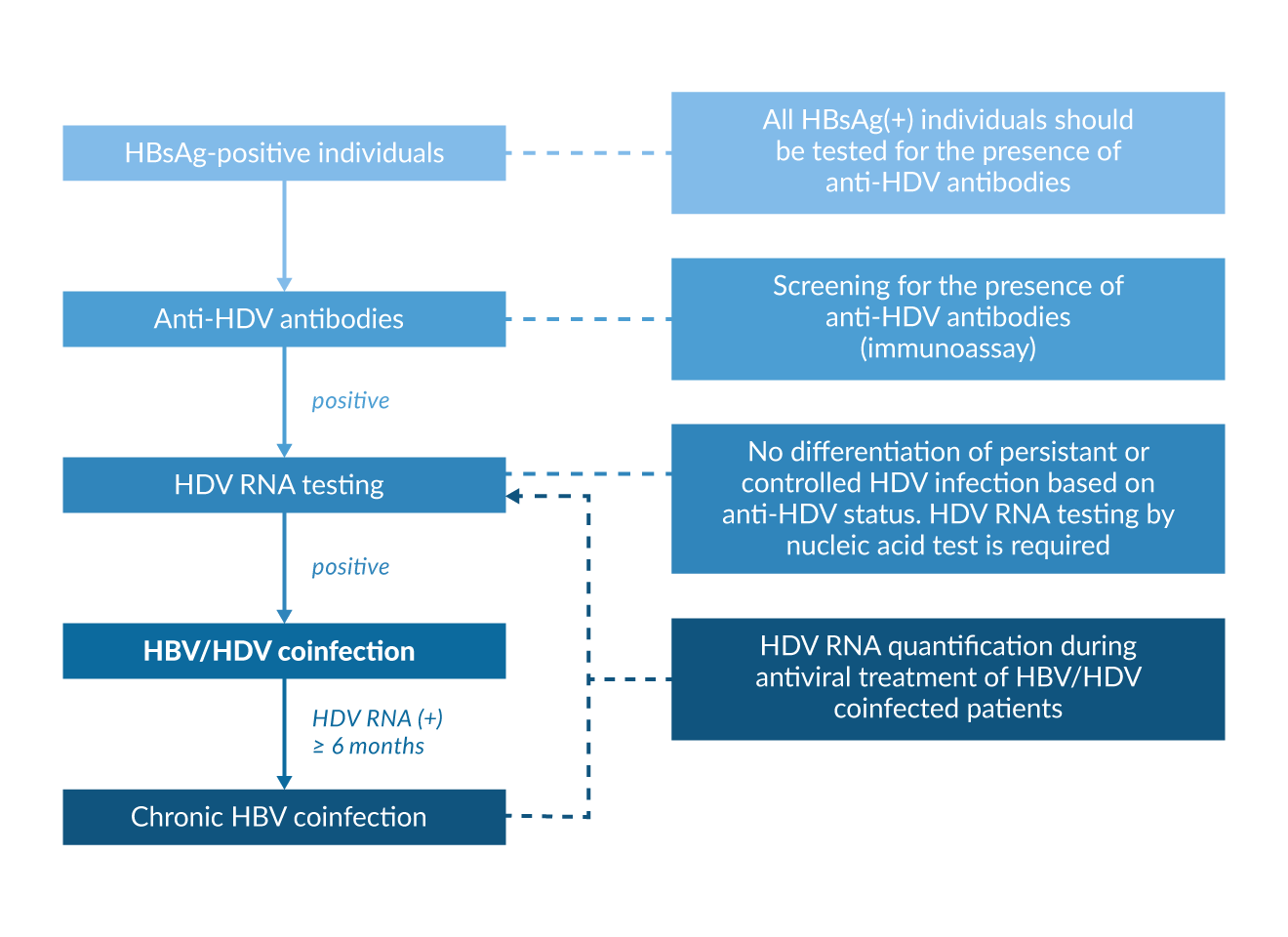 Figure 3. Diagnostic algorithm in HBsAg positive individuals
Figure 3. Diagnostic algorithm in HBsAg positive individuals
Treatment of HDV
With bulevirtide (BLV) and pegylated interferon alfa (PEG-IFN) two treatment options are currently available. Antiviral efficacy against HDV has been demonstrated in randomized trials for both compounds. Therefore, treatment options should be evaluated in all patients with chronic HDV infection and detectable HDV RNA. Patients with high levels of liver inflammation advanced liver fibrosis or liver cirrhosis should be prioritized for antiviral therapy (Sandmann 2023a). Due to the rarity of the disease, treatment in a hepatology center is recommended. This is especially true for patients with advanced liver disease as liver transplantation should also be considered for these patients (Sandmann 2023a). In general, BLV and PEG-IFN show different treatment modalities, side effect profile and response rates. For the choice of treatment, advantages and disadvantages of available treatment options should be weighed up and discussed with the patient (Table 2 and Table 3). A summary of treatment options is depicted in figure 4.
Table 2. Advantage and disadvantages of bulevirtide and pegylated interferon treatment (adapted from (8))| Advantages | Disadvantages | |
| Bulevirtide |
|
|
| Pegylated interferon alfa |
|
|
| Pegylated interferon alfa plus bulevirtide |
|
|
| Study | Cohorts | Combined response (≥2 log HDV RNA decline or negativity + ALT normali-zation) at EOT | Viro-logical response (≥2 log HDV RNA decline or nega-tivity) at EOT | HDV RNA nega-tivity at EOT | Bio-chemical response (ALT normali-zation) at EOT | HDV RNA nega-tivity at FU24 |
| MYR202 N=118 (Wedemeyer 2023c) | a) 2mg BLV plus TDF 24W (n=28) b) 5mg BLV plus TDF 24W (n=32) c) 10mg plus TDF 24W (n=30) d) TDF 24W (n=28) | a) 21% b) 28% c) 37% d) 0% | a) 54% b) 50% c) 77% d) 4% | a) 4% b) 6% c) 3% d) 0% | a) 43% b) 50% c) 40% d) 7% | a) 4% b) 3% c) 0% d) 0% |
| MYR301, n=150 (Wedemeyer 2023a) | a) No therapy 48W1 (n=51) b) 2mg BLV 48W1 (n=49) c) 10mg BLV 48W1 (n=50) All groups with or without TDF | a) 2% b) 45% c) 48% | a) 4% b) 71% c) 76% | a) 0% b) 12% c) 20% | a) 12% b) 51% c) 56% | n.a. |
| HIDIT-I, n=90 (Wedemeyer 2011) | a) PEG-IFN plus ADF 48W (31) b) PEG-IFN 48W (n=29) c) ADV 48W (n=30) | n.a. | a) 26%2 b) 31%2 c) 0%2 | a) 23% b) 24% c) 0% | a) 32% b) 28% c) 7% | a) 26% b) 31% c) 0% |
| HIDIT-II, n=120 (Wedemeyer 2019b) | a) PEG-IFN plus TDF 96W (n=59) b) PEG-IFN 96W (n=61) | n.a. | n.a. | a) 48% b) 33% | a) 44% b) 38% | a) 31% b) 23% |
ADF, adefovir; BLV, bulevirtide; EOT, end of treatment; FU24, follow-up 24 weeks (24 weeks after end of treatment); PEG-IFN, pegylated interferon alfa; TDF, tenofovir; W, weeks
Bulevirtide
Bulevirtide is the first drug for the treatment of chronic HDV infection that received approval by the European Medicines Agency (EMA 2024a). BLV is approved for the treatment of patients with chronic HDV infection, detectable HDV RNA and compensated liver disease (EMA 2024a). Treatment is administered subcutaneously once daily at a dose of 2 mg with or without concomitant nucleos(t)ide analog (NA) treatment. Currently, the optimal treatment duration is not known and treatment should be administered as long as there is a benefit for the patient.
BLV blocks the entry of HBV and HDV into hepatocytes by binding to and blocking NTCP, a bile salt transporter of the liver (Li 2016). Analyses of biopsy data from clinical trials have shown that BLV leads to a reduction in necroinflammation (Wedemeyer 2023c) and a reduction in HDV-infected hepatocytes, which correlates with a reduction in intrahepatic HDV RNA (Allweiss 2024). Due to the mechanism of action, patients treated with BLV show elevated bile acid levels, which has not been shown to be of clinical relevance (i.e. patients do not experience pruritus) (Wedemeyer 2023a).
BLV was approved on the basis of two phase 2 studies in which either BLV in combination with tenofovir (MYR202) or BLV monotherapy (MYR203) was carried out. The duration of therapy was 24 and 48 weeks, respectively. BLV therapy resulted in a decrease in HDV RNA, which, however, was reversible after the end of therapy. Recently, results of the ongoing phase 3 study, MYR301 have been published (Wedemeyer 2023a). The primary endpoint, combined response (HDV RNA decline or undetectability and ALT normalization) after 48 weeks of treatment, was significantly more frequent in patients receiving BLV 2 mg compared to patients without BLV treatment (45% vs. 2%, p<0.001). After 48 weeks of treatment, 12% of patients receiving BLV 2 mg showed HDV RNA undetectability and 51% of patients had normalized their ALT values (Table 3) (Wedemeyer 2023a). BLV treatment was overall well tolerated and no treatment interruptions due to side effects were registered. Furthermore, health related quality of life measured by Hepatitis Quality of Life Questionnaire improved significantly for patients receiving treatment (Buti 2022).
Results of the interim analysis after 96 weeks of treatment were recently published. With ongoing treatment duration, virological, biochemical and combined response rates further increased (Wedemeyer 2024) . Due to the conditional approval in 2020, case reports and case series from Europe have been published that show the use of bulevirtide in clinical practice. Treatment response rates were overall comparable to the ones from clinical trials. In July 2023, BLV received full approval by EMA.
Importantly, the proportion of patients with cirrhosis, even portal hypertension, was high in these real-world cohorts, emphasizing that bulevirtide can be safely used in (compensated) advanced cirrhosis (Degasperi 2022b, Dietz-Fricke 2023, Herta 2022, Jachs 2022, Zollner 2022). As of 4/2024, BLV is not approved in patients with decompensated liver disease. However, in the German real-world cohort, a total of 5 patient with decompensated liver cirrhosis (Child-Pugh B n=4, Child-Pugh C: n=1) were treated with BLV. ALT levels decreased and platelet counts increased in 4 patients and one patient with refractory ascites experienced transient improvement. One patient developed decompensation (ascites) during therapy, BLV was safely continued, and the cause of decompensation was attributed to another precipitating cause (Dietz-Fricke 2023). This is of particular importance as discontinuation of BLV therapy can lead to a rebound in HDV RNA and in patients with decompensated liver function there is concern that the rebound in HDV RNA could lead to a further deterioration of liver function. Therefore, if possible, treatment with BLV should be continued if decompensation occurs during therapy, especially if the HDV RNA is suppressed by the therapy.
In general, the treatment duration of BLV therapy is still unclear. Current guidelines recommend to continue treatment for as long as there is a benefit for the patient (EASL 2023). The phase 3 study (MYR301) is investigating the course after discontinuation of bulevirtide after a previous treatment duration of 96 to 144 weeks (Wedemeyer 2023a). These results are not yet available and must be awaited in order to assess whether a maintained response can be achieved after discontinuation of bulevirtide therapy for more than 96 weeks. Current real-world data show a rebound in HDV RNA after stopping bulevirtide, even after a treatment duration of more than 48 weeks (Jachs 2022). Re-treatment with BLV was successful in all cases and no resistances were detected (Jachs 2023). Nevertheless, some patients remained HDV RNA suppressed after treatment cessation even without achieving HBsAg loss (Anolli 2023, Jachs 2023). However, so far there are no stopping rules and due to the above-mentioned risk of deteriorating liver function, BLV should not be stopped in patients with decompensated liver disease. Maintained virological control has so far been shown in particular with the combination therapy PEG-IFN plus bulevirtide and HBsAg loss (Lampertico 2022).
The addition of PEG-IFN to bulevirtide therapy may in principle increase response rates, as the combination therapy may have synergistic effects. It has been shown in vitro, that interferon treatment inhibits cell-to-cell spread of HDV (Zhang 2022) thereby reducing the number of HDV-infected hepatocytes. The combination of PEG-IFN and BLV has been and is being investigated in clinical trials (Bogomolov 2016, Lampertico 2022). Data from the phase 2 study MYR204 has recently been published. The combination of PEG-IFN and BLV 2 mg showed similar off-treatment results compared to PEG-IFN monotherapy while the combination of PEG-IFN and BLV 10 mg achieved the highest rate of HDV RNA undetectability at 48 weeks after end of treatment (Asselah 2024). Data from the MYR203 study has only been presented as a congress paper (Wedemeyer 2019a) and further information is summarized in a review (Lampertico 2022). In addition, real-world data on the use of PEG-IFN plus BLV have been presented at congresses (De Ledinghen 2022, de Lédinghen 2022, Fontaine 2022) and published in small case series (Jachs 2022). With the limitation of heterogeneous treatment regimens, the overall data confirm the virological response rates and safety of PEG-IFN/BLV therapy reported in clinical trials (Lampertico 2022). Preliminary data from the French early access cohort show comparable data to BLV monotherapy in terms of combined response (HDV RNA decline ≥ 2 log plus ALT normalization) after 2 years of PEG-IFN/BLV therapy (De Ledinghen 2022). In an Austrian case series, combination therapy with PEG-IFN was initiated in patients who plateaued HDV RNA HDV RNA after 24-48 weeks of BLV therapy, regardless of initial response classification (Jachs 2022). It is currently unclear which patients will benefit from combination therapy. In addition, timing and duration of combination therapy are not known. It is unclear whether combination therapy should be given from the start or whether it should be started during the course of BLV monotherapy after certain criteria have been met. However, based on many years of experience with PEG-IFN therapy and the first real-world data, combination therapy with BLV plus PEG-IFN may be an option for experienced physicians treating hepatitis D in individual cases (EASL 2023, Sandmann 2023a).
Pegylated interferon alfa
Pegylated interferon alfa-2a (PEG-IFN) has antiviral activity against HDV, however, it is only approved for the treatment of hepatitis B (EMA 2024b). The specific mechanism of action of interferon alfa on HDV is still not fully understood. One effect of PEG-IFN treatment is the activation of the JAK-STAT pathway, which leads to transcription of interferon-stimulated genes, resulting in an "antiviral state." Importantly, in HDV infection, interferon alfa also suppresses cell division-mediated HDV spread by destabilizing HDV RNA during cell division (Zhang 2022). Interferon alfa therapy (standard or PEG-IFN) achieves up to 47% HDV RNA suppression, with the highest response rates documented in smaller cohort studies (Farci 1994, Sandmann 2023b). In the two large prospective randomized controlled HIDIT trials, the response rate in the PEG-IFN monotherapy groups was 23-33% at the end of treatment. At 24 weeks after end of treatment, 23-31% of patients had undetectable HDV RNA (Wedemeyer 2011, Wedemeyer 2019b) (Table 3). However, during long-term follow-up, late HDV RNA relapses were detected in 55% of the patients from the HIDIT-I study (Heidrich 2014, Wranke 2020). Therefore, unlike in hepatitis C, the term "sustained virlogical response" (SVR) should not be used and long-term follow-up is needed even after antiviral treatment has ended. Based on these studies, the long-term effects on clinical endpoints after PEG-IFN based treatment have been investigated, providing a solid data base for therapy with PEG-IFN.
Current treatment guidelines recommend a treatment duration of 48 weeks (EASL 2023, Sandmann 2023a). During treatment, regularly blood tests are warranted as a decrease in leukocytes and platelets is a common side-effect and dose adjustments might be necessary. Interferon treatment can induce autoimmune thyreopathy (Andrade 2011). Therefore, also TSH should be monitored before and during therapy.
Extension of treatment duration to 96 weeks was investigated in the HIDIT-II study (Wedemeyer 2019b). Longer treatment duration did not significantly increase the number of patients with maintained treatment response. Therefore, an extension of therapy beyond 48 weeks is not generally recommended. However, if a decrease in HBsAg levels is observed during treatment, continuation of treatment beyond 48 weeks may be reasonable as the goal of HBsAg loss may be achieved in some patients (Heller 2014, Hercun 2021). HBsAg loss defines functional cure of the underlying HBV infection and is associated with improved long-term clinical outcome (Cornberg 2020, Wranke 2017).
Predictors of response or nonresponse to PEG-IFN have only been studied retrospectively. Based on data from the HIDIT-I trial (Wedemeyer 2011) HDV RNA and HBsAg were analyzed as predictors of treatment response to PEG-IFN (with or without adefovir). Patients with ≥ 2 log HDV RNA decrease at treatment week 24 were at low risk for nonresponse at the end of therapy and negative HDV RNA at treatment week 24 or 48 proved to be an important prerequisite for treatment response 24 weeks after end of therapy. The best parameter for predicting nonresponse at the end of therapy was an HDV RNA decline < 1 log combined with no decline of HBsAg at treatment week 24 (positive predictive value of 83%) (Keskin 2015). Post-hoc analyses also exist for the HIDIT-II study (Wedemeyer 2019b). Here, low levels of hepatitis B core related antigen (HBcrAg) before treatment initiation and at week 24 of therapy were associated with treatment response 24 weeks after the end of therapy (Sandmann 2022). However, the data are not yet robust enough to define clear stopping rules for PEG-IFN-based therapies. It is important to be aware that that PEG-IFN-related side effects (flu-like symptoms, myelosuppression, psychiatric effects) limit PEG IFN-based treatment in some patient groups, and the therapy is contraindicated in advanced liver disease and decompensated liver cirrhosis. Nevertheless, synergistic effects of PEG-IFN with other drugs under development are conceivable because of its particular mechanism of action.
Nucleoside and nucleotide analogues
Nucleoside and nucleotide analogues (NA) used for the treatment of HBV infection have no direct antiviral effects against HDV as HDV uses host polymerases for replication. Several studies have shown the lack of efficacy of NA against HDV (Famciclovir (Yurdaydin 2002), lamivudine (Niro 2005), entecavir (Kabacam 2012) and adefovir (Wedemeyer 2011)). However, data from HIV/HBV/HDV-coinfected patients from Spain and Switzerland showed a decline of HDV RNA during treatment with tenofovir (TDF) (Beguelin 2017a, Soriano 2014). In the Spanish cohort, HDV RNA suppression to undetectable levels occurred in 10/19 patients after a median use of TDF of 58 months (Soriano 2014). It is interesting to note that HDV RNA declines were not associated with HBsAg declines In the SWISS HIV cohort, TDF-containing ART was associated with relevant HDV RNA declines in 29% of patients and 14% had undetectable HDV RNA after 5 years (Beguelin 2017a). One hypothesis is that TDF may induce interferon lambda (Murata 2020) which has been shown to exert also direct antiviral effects against HDV (Giersch 2017). However, TDF in combination PEG-IFN showed no additional effect compared with PEG-IFN alone in the treatment of HBV/HDV coinfected patients (Wedemeyer 2019b). Another hypothesis is the improvement of host immunity that has been compromised by HIV through the effective treatment of antiretroviral therapy, which includes TDF.
Additionally, retrospective studies investigated the clinical course of patients receiving NA treatment. In these studies, outcomes were worse with NA alone compared to PEG-IFN treatment. However, selection bias should be considered here since NA monotherapy was usually used in patients with contraindication against PEG-IFN, e.g. decompensated liver disease (Kamal 2020, Wranke 2017).
To what extend liver disease progression due to hepatitis B viremia can be reduced by suppression of HBV DNA in HDV coinfected patients is elusive. Still, it can be assumed that the therapeutic principles that have been established in HBV monoinfection can also be applied in coinfection with HDV (EASL 2023). Therefore, in daily practice, the same treatment indications apply to HBV viremia in chronic HDV infection as to HBV monoinfection (EASL 2017). Importantly, patients with liver cirrhosis and detectable HBV DNA should receive NA treatment with entecavir or tenofovir (EASL 2023, Sandmann 2023a).
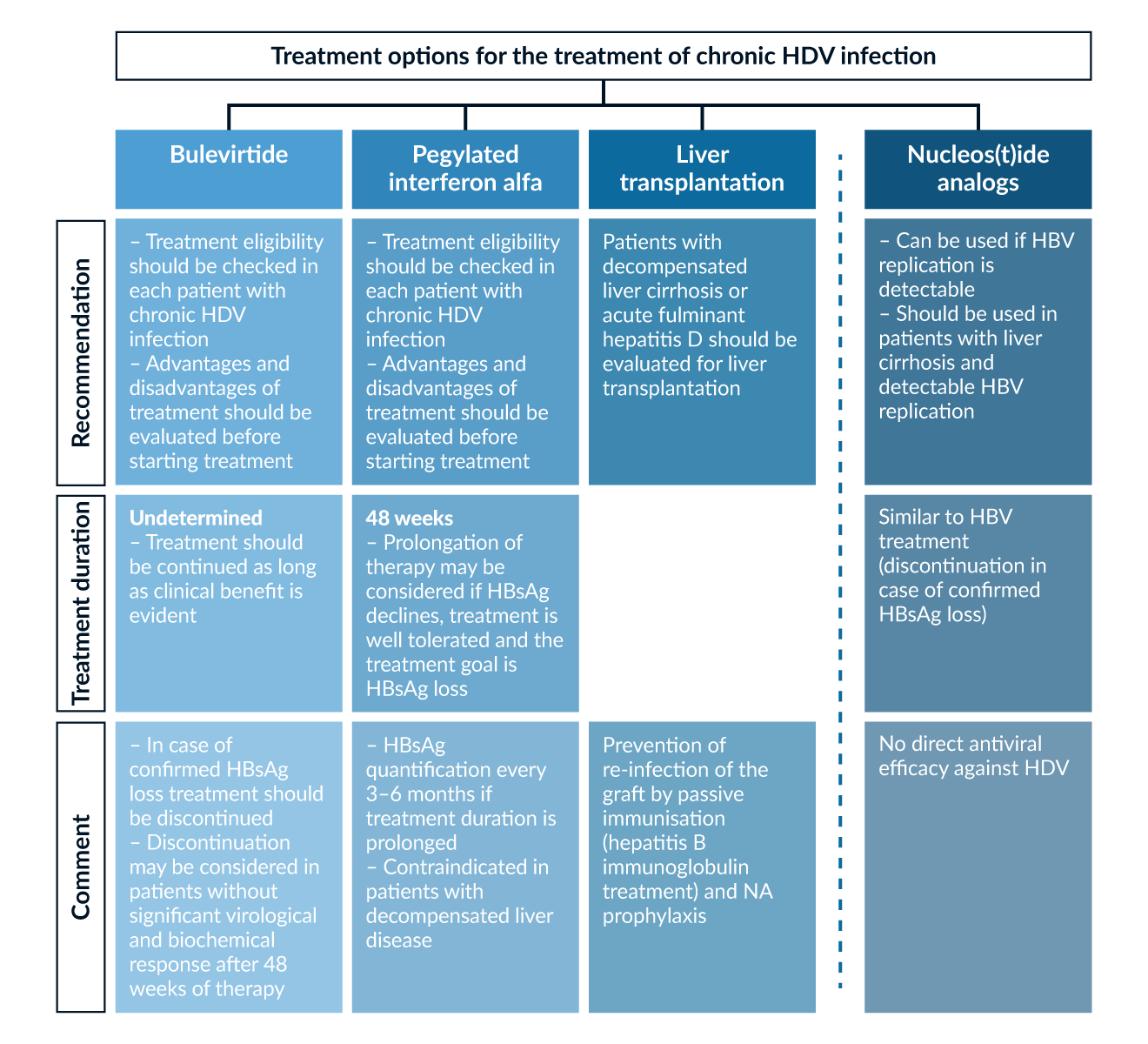 Figure 4. Treatment options for the treatment of chronic HDV infection (EASL 2017, 2023, Sandmann 2023a)
Figure 4. Treatment options for the treatment of chronic HDV infection (EASL 2017, 2023, Sandmann 2023a)
New drugs against HDV in clinical development
The prenylation of the large delta antigen is essential for virus particle formation. The prenylation inhibitor lonafarnib (LNF) showed a dose-dependent reduction of HDV RNA levels of up to 2 log IU/mL after 28 days of therapy (Yurdaydin 2018b). Importantly, HDV RNA declines were associated with LNF serum concentrations. While there was no evidence for viral resistance, higher doses of LFN caused nausea and diarrhea in most patients. Therefore, boostering with ritonavir was introduced in later clinical trials (Eiger 2023a). The phase 3 study is currently investigating the combination of LNF plus ritonavir (LFN/r) with or without PEG-IFN in chronic HDV patients receiving NA maintenance therapy. After 48 weeks of treatment, LNF/r and LFN/r plus PEG-IFN achieved the primary endpoint of virological and biochemical response in 12.6% and 24.2% of patients, respectively. Moreover, the combination arm showed statistically significant histological improvement (Etzion 2023a). Recently, follow-up 72-week data presented at the EASL 2023 meeting revealed that the combination still showed consistent endpoint response and that the treatment was well tolerated in both arms.
Nucleic acid polymers are being developed to treat patients with HDV (Bazinet 2017). Rep 2139-Ca is believed to block the release of subviral HBsAg particles from hepatocytes. The compound was injected once weekly and induced a marked decline of HBsAg in some but not all patients with HDV treated in a center in Moldova. Of note, all patients treated (n=12) showed an HDV RNA decline after 15 weeks of monotherapy when PEG-IFN was added. Responses were maintained in seven patients one year after completing treatment. A transient ALT increase was observed in patients with low HBsAg levels after REP 2139 monotherapy when PEG-IFN was introduced. In addition, several case reports from a Compassionate Use program have been presented at meetings confirming responses (HDV RNA decline and also HBsAg loss in some patients) observed in the trial from Moldavia (Stern 2023). Future studies will need to determine the efficacy and safety of REP 2139 in a larger group of patients with HDV infection.
Interferon lambda was also explored in patients with HDV infection, both as a monotherapy or in combination with LNF (Etzion 2023b, Sandmann 2021). In vitro and in humanized mice, an antiviral effect comparable to interferon alpha has been observed (Giersch 2017). The potential advantage of interferon lambda is the lower frequency of systemic side effects as compared to interferon alfa. However, the further development was recently stopped due to ALT flares in some patients that resulted in liver decompensation (Eiger 2023b).
Last but not least, monoclonal antibodies against HBsAg with neutralizing activity, as well as RNA interfering drugs (ASO, siRNA), have entered clinical evaluation. However, additional research is needed to validate their use in larger trials and real-world clinical settings (Sandmann 2021).
Liver transplantation for HDV
Liver transplantation remains the ultimate treatment option for many patients with chronic hepatitis D with end-stage liver disease. If prophylaxis by passive immunization with anti-HBs antibodies (hepatitis B immunoglobulins, HBIG) and administration of NA prophylaxis is applied, HBV/HDV reinfection can be prevented in all individuals (Rosenau 2007) leading to an excellent long-term outcome after transplantation. HDV RNA levels rapidly decline during the first days after transplantation (Mederacke 2012) but HDVAg may persist in the transplanted liver for several years (Mederacke 2012, Smedile 1998). The possibility of reactivation of latent HDV infection by HBV superinfection has also been confirmed experimentally in a mouse model with transplanted human hepatocytes (Giersch 2014). Mice infected with HDV lacking HBV could be rescued by HBV superinfection after 2-6 weeks leading to a productive coinfection. Long-term prophylaxis to prevent HBV reinfection is therefore generally recommended in patients transplanted for HDV as reinfection may lead to HDV reactivation for which treatment options are very limited. Still, two recent reports challenge the current practice of dual prophylaxis as only 2 out of 34 and 1 out of 17 patients had HBV/HDV recurrence when administration of HBV immunoglobulins was stopped after transplantation (Cholongitas 2016, Ossami Saidy 2021). Furthermore, HDV recurrence was not observed after HBIG discontinuation in 64 cases that were separately reported from different groups (Caccamo 2017, Fernandez 2015, Lenci 2023, Manini 2018, Ocal 2015). However, due to the small risk of HBV/HBV recurrence and the present limited treatment options, HBIG discontinuation is not recommended by current guidelines (EASL 2023, Sandmann 2023a). Since evidence on HBIG discontinuation after one to two years after liver transplantation is accumulating, there is the need to address the safety of this approach as part of future clinical trials.
Summary and outlook
Chronic infection with the hepatitis D virus (HDV) is rare, but represents a severe form of chronic liver disease. Immunopathogenesis plays an essential role in the control or progression of the infection. As treatment options are available with bulevirtide and pegylated interferon alfa, early identification of infected patients is important. Therefore, all HBsAg-positive patients should be tested for HDV and risk groups, i.e. intravenous drug use, migration from countries with high HDV prevalence, should be tested repeatedly. Antiviral treatment should be evaluated in all individuals with chronic HDV infection, with priority given to patients with high inflammatory activity and advanced fibrosis and cirrhosis. For treatment decision, the advantages and disadvantages of current treatment options should be weighed against each other. Treatment with PEG-IFN is finite, may result in HBsAg loss in some patients, but is associated with side effects and cannot be used in the presence of advanced liver disease or autoimmune disease. Bulevirtide is well tolerated, leads to HDV RNA suppression and ALT normalization in a large proportion of patients, but the duration of treatment is not yet defined. Liver transplantation is a remaining option when antiviral treatment is no longer possible or in the setting of hepatic decompensation or hepatocellular carcinoma. Further promising therapy concepts are currently being developed with the aim of achieving HDV cure. These ongoing developments hold the promise of providing more effective and comprehensive care for individuals affected by HDV in the near future.
References
Abbas Z. 2012. Hepatitis d in pakistan. J Coll Physicians Surg Pak. 22(9):547-548.
Abeywickrama-Samarakoon N, Cortay JC, Sureau C, Muller S, Alfaiate D, Guerrieri F et al. 2020. Hepatitis delta virus histone mimicry drives the recruitment of chromatin remodelers for viral rna replication. Nat Commun. 11(1):419.
Alfaiate D, Clément S, Gomes D, Goossens N, Negro F. 2020. Chronic hepatitis d and hepatocellular carcinoma: A systematic review and meta-analysis of observational studies. Journal of Hepatology. 73(3):533-539.
Alfaiate D, Lucifora J, Abeywickrama-Samarakoon N, Michelet M, Testoni B, Cortay JC et al. 2016. Hdv rna replication is associated with hbv repression and interferon-stimulated genes induction in super-infected hepatocytes. Antiviral Res. 136:19-31.
Allweiss L, Volmari A, Suri V, Wallin JJ, Flaherty JF, Manuilov D et al. 2024. Blocking viral entry with bulevirtide reduces the number of hdv-infected hepatocytes in human liver biopsies. J Hepatol.
Andernach IE, Leiss LV, Tarnagda ZS, Tahita MC, Otegbayo JA, Forbi JC et al. 2014. Characterization of hepatitis delta virus in sub-saharan africa. J Clin Microbiol. 52(5):1629-1636.
Andrade LJ, D'Oliveira A, Jr., Silva CA, Nunes P, Franca LS, Malta AM et al. 2011. A meta-analysis of patients with chronic hepatitis c treated with interferon-alpha to determine the risk of autoimmune thyroiditis. Acta Gastroenterol Latinoam. 41(2):104-110.
Anolli MP, Degasperi E, Allweiss L, Sangiovanni A, Maggioni M, Scholtes C et al. 2023. A 3-year course of bulevirtide monotherapy may cure hdv infection in cirrhotics. J Hepatol.
Asselah T, Chulanov V, Lampertico P, Wedemeyer H, Streinu-Cercel A, Pantea V et al. 2024. Bulevirtide combined with pegylated interferon for chronic hepatitis d. N Engl J Med. 391(2):133-143.
Asselah T, Rizzetto M. 2023. Hepatitis d virus infection. N Engl J Med. 389(1):58-70.
Bazinet M, Pântea V, Cebotarescu V, Cojuhari L, Jimbei P, Albrecht J et al. 2017. Safety and efficacy of rep 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis b virus and hepatitis d virus co-infection (rep 301 and rep 301-ltf): A non-randomised, open-label, phase 2 trial. The Lancet Gastroenterology & Hepatology. 2(12):877-889.
Beguelin C, Friolet N, Moradpour D, Sahli R, Suter-Riniker F, Luthi A et al. 2017a. Impact of tenofovir on hepatitis delta virus replication in the swiss human immunodeficiency virus cohort study. Clin Infect Dis. 64(9):1275-1278.
Beguelin C, Moradpour D, Sahli R, Suter-Riniker F, Luthi A, Cavassini M et al. 2017b. Hepatitis delta-associated mortality in hiv/hbv-coinfected patients. J Hepatol. 66(2):297-303.
Benegiamo G, Vinciguerra M, Guarnieri V, Niro GA, Andriulli A, Pazienza V. 2013. Hepatitis delta virus induces specific DNA methylation processes in huh-7 liver cancer cells. FEBS Lett. 587(9):1424-1428.
Bogomolov P, Alexandrov A, Voronkova N, Macievich M, Kokina K, Petrachenkova M et al. 2016. Treatment of chronic hepatitis d with the entry inhibitor myrcludex b: First results of a phase ib/iia study. J Hepatol. 65(3):490-498.
Braga WS, de Oliveira CM, de Araujo JR, Castilho Mda C, Rocha JM, Gimaque JB et al. 2014. Chronic hdv/hbv co-infection: Predictors of disease stage---a case series of hdv-3 patients. J Hepatol. 61(6):1205-1211.
Bremer B, Anastasiou OE, Ciesek S, Wedemeyer H. 2019. Automated nucleic acid isolation methods for hdv viral load quantification can lead to viral load underestimation. Antivir Ther. 24(2):117-123.
Buti M, Homs M, Rodriguez-Frias F, Funalleras G, Jardi R, Sauleda S et al. 2011. Clinical outcome of acute and chronic hepatitis delta over time: A long-term follow-up study. J Viral Hepat. 18(6):434-442.
Buti M, Wedemeyer H, Aleman S, Chulanov V, Viacheslav M, Sagalova O et al. 2022. Treatment with bulevirtide improves patient-reported outcomes in patients with chronic hepatitis delta: An exploratory analysis of a phase 3 trial at 48 weeks. Journal of Hepatology. 77:S103.
Caccamo L. 2017. Long-term nucleos(t)ide analog(s) monoprophylaxis in delta coinfected liver transplant recipients. Transpl Infect Dis. 19(1).
Calle Serrano B, Grosshennig A, Homs M, Heidrich B, Erhardt A, Deterding K et al. 2014. Development and evaluation of a baseline-event-anticipation score for hepatitis delta. J Viral Hepat. 21(11):e154-163.
Calle Serrano B, Manns MP, Wedemeyer H. 2012. Hepatitis delta and hiv infection. Semin Liver Dis. 32(2):120-129.
Casey JL, Niro GA, Engle RE, Vega A, Gomez H, McCarthy M et al. 1996. Hepatitis b virus (hbv)/hepatitis d virus (hdv) coinfection in outbreaks of acute hepatitis in the peruvian amazon basin: The roles of hdv genotype iii and hbv genotype f. J Infect Dis. 174(5):920-926.
Castellares C, Barreiro P, Martin-Carbonero L, Labarga P, Vispo ME, Casado R et al. 2008. Liver cirrhosis in hiv-infected patients: Prevalence, aetiology and clinical outcome. J Viral Hepat. 15(3):165-172.
Cholongitas E, Goulis I, Antoniadis N, Fouzas I, Imvrios G, Giakoustidis D et al. 2016. Nucleos(t)ide analog(s) prophylaxis after hepatitis b immunoglobulin withdrawal against hepatitis b and d recurrence after liver transplantation. Transpl Infect Dis. 18(5):667-673.
Cornberg M, Lok AS, Terrault NA, Zoulim F, Faculty E-AHTEC. 2020. Guidance for design and endpoints of clinical trials in chronic hepatitis b - report from the 2019 easl-aasld hbv treatment endpoints conference(double dagger). J Hepatol. 72(3):539-557.
Da BL, Surana P, Takyar V, Kleiner DE, Heller T, Koh C. 2020. Vibration-controlled transient elastography for the detection of cirrhosis in chronic hepatitis d infection. J Viral Hepat. 27(4):428-436.
De Ledinghen V, Hermabessière P, Metivier S, Bardou Jacquet E, Hilleret M-N, Loustaud Ratti V et al. 2022. Bulevirtide, with or without peg- interferon, in hdv infected patients in a real-life setting. Two-year results from the french multicenter early access program. Hepatology. 76:S26-S28.
de Lédinghen V, Metivier S, Bardou-Jacquet E, Hilleret M-N, Loustaud-Ratti V, Ganne-Carrié N et al. 2022. Treatment with bulevirtide in patients with chronic hbv/hdv co-infection. Safety and efficacy at month 18 in real-world settings. Journal of Hepatology. 77:S840-S840.
Degasperi E, Anolli MP, Sara C, Renteria U, Sambarino D, Borghi M et al. 2022a. Bulevirtide monotherapy for 48 weeks in hdv patients with compensated cirrhosis and clinically significant portal hypertension. Journal of Hepatology. 77:S868-S868.
Degasperi E, Anolli MP, Uceda Renteria SC, Sambarino D, Borghi M, Perbellini R et al. 2022b. Bulevirtide monotherapy for 48 weeks in patients with hdv-related compensated cirrhosis and clinically significant portal hypertension. J Hepatol. 77(6):1525-1531.
Degertekin H, Yalcin K, Yakut M, Yurdaydin C. 2008. Seropositivity for delta hepatitis in patients with chronic hepatitis b and liver cirrhosis in turkey: A meta-analysis. Liver Int. 28(4):494-498.
Deny P. 2006. Hepatitis delta virus genetic variability: From genotypes i, ii, iii to eight major clades? Curr Top Microbiol Immunol. 307:151-171.
Dias J, Hengst J, Parrot T, Leeansyah E, Lunemann S, Malone DFG et al. 2019. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of mait cells. J Hepatol. 71(2):301-312.
Dienes HP, Purcell RH, Popper H, Ponzetto A. 1990. The significance of infections with two types of viral hepatitis demonstrated by histologic features in chimpanzees. J Hepatol. 10(1):77-84.
Dietz-Fricke C, Tacke F, Zollner C, Demir M, Schmidt HH, Schramm C et al. 2023. Treating hepatitis d with bulevirtide - real-world experience from 114 patients. JHEP Rep. 5(4):100686.
EASL (European Association for the Study of the Liver). 2017. Easl 2017 clinical practice guidelines on the management of hepatitis b virus infection. J Hepatol. 67(2):370-398.
EASL (European Association for the Study of the Liver). 2023. Easl clinical practice guidelines on hepatitis delta virus. J Hepatol. 79(2):433-460.
Eiger 2023a. [accessed August 24, 2023]. https://clinicaltrials.gov/study/NCT03719313.
Eiger 2023b. [accessed accessed August 24, 2023]. https://ir.eigerbio.com/news-releases/news-release-details/eiger-discontinue-phase-3-limt-2-trial-peginterferon-lambda.
El Bouzidi K, Elamin W, Kranzer K, Irish DN, Ferns B, Kennedy P et al. 2015. Hepatitis delta virus testing, epidemiology and management: A multicentre cross-sectional study of patients in london. J Clin Virol. 66:33-37.
EMA (European Medicines Agency). Product information hepcludex. 2024a. [accessed 2024]. https://www.ema.europa.eu/en/documents/product-information/hepcludex-epar-product-information_de.pdf.
EMA (European Medicines Agency). Product information pegasys. 2024b. [accessed 2024]. https://www.ema.europa.eu/en/documents/product-information/pegasys-epar-product-information_de.pdf.
Erhardt A, Hoernke M, Heinzel-Pleines U, Sagir A, Gobel T, Haussinger D. 2010. Retrospective analysis of chronic hepatitis d in a west german university clinic over two decades: Migratory pattern, prevalence and clinical outcome. Z Gastroenterol. 48(8):813-817.
Etzion O, Hamid S, Asselah T, et al. 2023a. Gs-012 - week 48 results of the phase 3 d-livr study, a randomized double-blind, placebo-controlled trial evaluating the safety and efficacy of lonafarnib-boosted with ritonavir with or without peginterferon alfa in patients with chronic hepatitis delta. Journal of Hepatology. 78(S10).
Etzion O, Hamid S, Lurie Y, Gane EJ, Yardeni D, Duehren S et al. 2023b. Treatment of chronic hepatitis d with peginterferon lambda-the phase 2 limt-1 clinical trial. Hepatology. 77(6):2093-2103.
Farci P, Mandas A, Coiana A, Lai ME, Desmet V, Van Eyken P et al. 1994. Treatment of chronic hepatitis d with interferon alfa-2a. N Engl J Med. 330(2):88-94.
Farci P, Roskams T, Chessa L, Peddis G, Mazzoleni AP, Scioscia R et al. 2004. Long-term benefit of interferon alpha therapy of chronic hepatitis d: Regression of advanced hepatic fibrosis. Gastroenterology. 126(7):1740-1749.
Fernandez-Montero JV, Vispo E, Barreiro P, Sierra-Enguita R, de Mendoza C, Labarga P et al. 2014. Hepatitis delta is a major determinant of liver decompensation events and death in hiv-infected patients. Clin Infect Dis. 58(11):1549-1553.
Fernandez I, Loinaz C, Hernandez O, Abradelo M, Manrique A, Calvo J et al. 2015. Tenofovir/entecavir monotherapy after hepatitis b immunoglobulin withdrawal is safe and effective in the prevention of hepatitis b in liver transplant recipients. Transpl Infect Dis. 17(5):695-701.
Flodgren E, Bengtsson S, Knutsson M, Strebkova EA, Kidd AH, Alexeyev OA et al. 2000. Recent high incidence of fulminant hepatitis in samara, russia: Molecular analysis of prevailing hepatitis b and d virus strains. J Clin Microbiol. 38(9):3311-3316.
Fontaine H, Fougerou-Leurent C, Gordien E, Scholtes C, Metivier S, de Lédinghen V et al. 2022. Real life study of bulevirtide in chronic hepatitis delta: Preliminary results of the anrs hd ep01 buledelta prospective cohort. Journal of Hepatology. 77:S72-S72.
Gaeta GB, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, Lobello S et al. 2000. Chronic hepatitis d: A vanishing disease? An italian multicenter study. Hepatology. 32(4 Pt 1):824-827.
Gheorghe L, Csiki IE, Iacob S, Gheorghe C, Trifan A, Grigorescu M et al. 2015. Hepatitis delta virus infection in romania: Prevalence and risk factors. J Gastrointestin Liver Dis. 24(4):413-421.
Giersch K, Allweiss L, Volz T, Helbig M, Bierwolf J, Lohse AW et al. 2015. Hepatitis delta co-infection in humanized mice leads to pronounced induction of innate immune responses in comparison to hbv mono-infection. J Hepatol. 63(2):346-353.
Giersch K, Helbig M, Volz T, Allweiss L, Mancke LV, Lohse AW et al. 2014. Persistent hepatitis d virus mono-infection in humanized mice is efficiently converted by hepatitis b virus to a productive co-infection. J Hepatol. 60(3):538-544.
Giersch K, Homs M, Volz T, Helbig M, Allweiss L, Lohse AW et al. 2017. Both interferon alpha and lambda can reduce all intrahepatic hdv infection markers in hbv/hdv infected humanized mice. Sci Rep. 7(1):3757.
Grabowski J, Wedemeyer H. 2010. Hepatitis delta: Immunopathogenesis and clinical challenges. Dig Dis. 28(1):133-138.
Grabowski J, Yurdaydin C, Zachou K, Buggisch P, Hofmann WP, Jaroszewicz J et al. 2011. Hepatitis d virus-specific cytokine responses in patients with chronic hepatitis delta before and during interferon alfa-treatment. Liver Int. 31(9):1395-1405.
Han M, Littlejohn M, Yuen L, Edwards R, Devi U, Bowden S et al. 2014. Molecular epidemiology of hepatitis delta virus in the western pacific region. J Clin Virol. 61(1):34-39.
He W, Cao Z, Mao F, Ren B, Li Y, Li D et al. 2016. Modification of three amino acids in sodium taurocholate cotransporting polypeptide renders mice susceptible to infection with hepatitis d virus in vivo. J Virol. 90(19):8866-8874.
He W, Ren B, Mao F, Jing Z, Li Y, Liu Y et al. 2015. Hepatitis d virus infection of mice expressing human sodium taurocholate co-transporting polypeptide. PLoS Pathog. 11(4):e1004840.
Heidrich B, Deterding K, Tillmann HL, Raupach R, Manns MP, Wedemeyer H. 2009. Virological and clinical characteristics of delta hepatitis in central europe. J Viral Hepat. 16(12):883-894.
Heidrich B, Serrano BC, Idilman R, Kabacam G, Bremer B, Raupach R et al. 2012. Hbeag-positive hepatitis delta: Virological patterns and clinical long-term outcome. Liver Int. 32(9):1415-1425.
Heidrich B, Yurdaydin C, Kabacam G, Ratsch BA, Zachou K, Bremer B et al. 2014. Late hdv rna relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 60(1):87-97.
Heller T, Rotman Y, Koh C, Clark S, Haynes-Williams V, Chang R et al. 2014. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 40(1):93-104.
Hercun J, Kim GE, Da BL, Rotman Y, Kleiner DE, Chang R et al. 2021. Durable virological response and functional cure of chronic hepatitis d after long-term peginterferon therapy. Aliment Pharmacol Ther. 54(2):176-182.
Herta T, Hahn M, Maier M, Fischer J, Niemeyer J, Honemann M et al. 2022. Efficacy and safety of bulevirtide plus tenofovir disoproxil fumarate in real-world patients with chronic hepatitis b and d co-infection. Pathogens. 11(5).
Hoblos R, Kefalakes H. 2023. Immunology of hepatitis d virus infection: General concepts and present evidence. Liver Int. 43 Suppl 1:47-59.
Homs M, Rodriguez-Frias F, Gregori J, Ruiz A, Reimundo P, Casillas R et al. 2016. Evidence of an exponential decay pattern of the hepatitis delta virus evolution rate and fluctuations in quasispecies complexity in long-term studies of chronic delta infection. PLoS One. 11(6):e0158557.
Huang YH, Tao MH, Hu CP, Syu WJ, Wu JC. 2004. Identification of novel hla-a*0201-restricted cd8+ t-cell epitopes on hepatitis delta virus. J Gen Virol. 85(Pt 10):3089-3098.
Hughes SA, Wedemeyer H, Harrison PM. 2011. Hepatitis delta virus. Lancet. 378(9785):73-85.
Hung CC, Wu SM, Lin PH, Sheng WH, Yang ZY, Sun HY et al. 2014. Increasing incidence of recent hepatitis d virus infection in hiv-infected patients in an area hyperendemic for hepatitis b virus infection. Clin Infect Dis. 58(11):1625-1633.
Jachs M, Panzer M, Hartl L, Schwarz M, Balcar L, Camp JV et al. 2023. Long-term follow-up of patients discontinuing bulevirtide treatment upon long-term hdv-rna suppression. JHEP Rep. 5(8):100751.
Jachs M, Schwarz C, Panzer M, Binter T, Aberle SW, Hartl L et al. 2022. Response-guided long-term treatment of chronic hepatitis d patients with bulevirtide-results of a "real world" study. Aliment Pharmacol Ther. 56(1):144-154.
Kabacam G, Onder FO, Yakut M, Seven G, Karatayli SC, Karatayli E et al. 2012. Entecavir treatment of chronic hepatitis d. Clin Infect Dis. 55(5):645-650.
Kamal H, Fornes R, Simin J, Stal P, Duberg AS, Brusselaers N et al. 2021. Risk of hepatocellular carcinoma in hepatitis b and d virus co-infected patients: A systematic review and meta-analysis of longitudinal studies. J Viral Hepat. 28(10):1431-1442.
Kamal H, Westman G, Falconer K, Duberg AS, Weiland O, Haverinen S et al. 2020. Long-term study of hepatitis delta virus infection at secondary care centers: The impact of viremia on liver-related outcomes. Hepatology. 72(4):1177-1190.
Karatayli SC, Bozdayi M, Karatayli E, Ozturk T, Husseini AA, Albayrak R et al. 2015. Interleukin-28 gene polymorphisms may contribute to hbsag persistence and the development of hbeag-negative chronic hepatitis b. Liver Int. 35(3):846-853.
Karimzadeh H, Kiraithe MM, Kosinska AD, Glaser M, Fiedler M, Oberhardt V et al. 2018. Amino acid substitutions within hla-b*27-restricted t cell epitopes prevent recognition by hepatitis delta virus-specific cd8(+) t cells. J Virol. 92(13).
Karimzadeh H, Kiraithe MM, Oberhardt V, Salimi Alizei E, Bockmann J, Schulze Zur Wiesch J et al. 2019. Mutations in hepatitis d virus allow it to escape detection by cd8(+) t cells and evolve at the population level. Gastroenterology. 156(6):1820-1833.
Kay A, Melo da Silva E, Pedreira H, Negreiros S, Lobato C, Braga W et al. 2014. Hbv/hdv co-infection in the western brazilian amazonia: An intriguing mutation among hdv genotype 3 carriers. J Viral Hepat. 21(12):921-924.
Kefalakes H, Horgan XJ, Jung MK, Amanakis G, Kapuria D, Bolte FJ et al. 2021. Liver-resident bystander cd8(+) t cells contribute to liver disease pathogenesis in chronic hepatitis d virus infection. Gastroenterology. 161(5):1567-1583 e1569.
Kefalakes H, Koh C, Sidney J, Amanakis G, Sette A, Heller T et al. 2019. Hepatitis d virus-specific cd8(+) t cells have a memory-like phenotype associated with viral immune escape in patients with chronic hepatitis d virus infection. Gastroenterology. 156(6):1805-1819 e1809.
Keskin O, Wedemeyer H, Tuzun A, Zachou K, Deda X, Dalekos GN et al. 2015. Association between level of hepatitis d virus rna at week 24 of pegylated interferon therapy and outcome. Clin Gastroenterol Hepatol. 13(13):2342-2349 e2341-2342.
Khodjaeva M, Ibadullaeva N, Khikmatullaeva A, Joldasova E, Ismoilov U, Colombo M et al. 2019. The medical impact of hepatitis d virus infection in uzbekistan. Liver Int. 39(11):2077-2081.
Kucirka LM, Farzadegan H, Feld JJ, Mehta SH, Winters M, Glenn JS et al. 2010. Prevalence, correlates, and viral dynamics of hepatitis delta among injection drug users. J Infect Dis. 202(6):845-852.
Kushner T, Serper M, Kaplan DE. 2015. Delta hepatitis within the veterans affairs medical system in the united states: Prevalence, risk factors, and outcomes. J Hepatol. 63(3):586-592.
Lampertico P, Roulot D, Wedemeyer H. 2022. Bulevirtide with or without pegifnalpha for patients with compensated chronic hepatitis delta: From clinical trials to real-world studies. J Hepatol. 77(5):1422-1430.
Le Gal F, Brichler S, Drugan T, Alloui C, Roulot D, Pawlotsky J-M et al. 2017. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology (Baltimore, Md). 66(6):1826-1841.
Le Gal F, Brichler S, Sahli R, Chevret S, Gordien E. 2016. First international external quality assessment for hepatitis delta virus rna quantification in plasma. Hepatology. 64(5):1483-1494.
Lee CY, Tsai HC, Lee SS, Wu KS, Sy CL, Chen JK et al. 2015. Higher rate of hepatitis events in patients with human immunodeficiency virus, hepatitis b, and hepatitis d genotype ii infection: A cohort study in a medical center in southern taiwan. J Microbiol Immunol Infect. 48(1):20-27.
Lenci I, Tariciotti L, Angelico R, Milana M, Signorello A, Manzia TM et al. 2023. Successful clinical and virological outcomes of liver transplantation for hdv/hbv-related disease after long-term discontinuation of hepatitis b immunoglobulins. Clin Transplant. 37(6):e14971.
Li W, Urban S. 2016. Entry of hepatitis b and hepatitis d virus into hepatocytes: Basic insights and clinical implications. J Hepatol. 64(1 Suppl):S32-S40.
Liao B, Zhang F, Lin S, He H, Liu Y, Zhang J et al. 2014. Epidemiological, clinical and histological characteristics of hbv/hdv co-infection: A retrospective cross-sectional study in guangdong, china. PLoS One. 9(12):e115888.
Lin HH, Lee SS, Yu ML, Chang TT, Su CW, Hu BS et al. 2015. Changing hepatitis d virus epidemiology in a hepatitis b virus endemic area with a national vaccination program. Hepatology. 61(6):1870-1879.
Lunemann S, Malone DF, Grabowski J, Port K, Beziat V, Bremer B et al. 2015. Effects of hdv infection and pegylated interferon alpha treatment on the natural killer cell compartment in chronically infected individuals. Gut. 64(3):469-482.
Lunemann S, Malone DF, Hengst J, Port K, Grabowski J, Deterding K et al. 2014. Compromised function of natural killer cells in acute and chronic viral hepatitis. J Infect Dis. 209(9):1362-1373.
Lutgehetmann M, Mancke LV, Volz T, Helbig M, Allweiss L, Bornscheuer T et al. 2012. Humanized chimeric upa mouse model for the study of hepatitis b and d virus interactions and preclinical drug evaluation. Hepatology. 55(3):685-694.
Lutterkort GL, Wranke A, Yurdaydin C, Budde E, Westphal M, Lichtinghagen R et al. 2017. Non-invasive fibrosis score for hepatitis delta. Liver Int. 37(2):196-204.
Manesis EK, Vourli G, Dalekos G, Vasiliadis T, Manolaki N, Hounta A et al. 2013. Prevalence and clinical course of hepatitis delta infection in greece: A 13-year prospective study. J Hepatol. 59(5):949-956.
Manini MA, Whitehouse G, Bruce M, Passerini M, Lim TY, Carey I et al. 2018. Entecavir or tenofovir monotherapy prevents hbv recurrence in liver transplant recipients: A 5-year follow-up study after hepatitis b immunoglobulin withdrawal. Dig Liver Dis. 50(9):944-953.
Mederacke I, Filmann N, Yurdaydin C, Bremer B, Puls F, Zacher BJ et al. 2012. Rapid early hdv rna decline in the peripheral blood but prolonged intrahepatic hepatitis delta antigen persistence after liver transplantation. J Hepatol. 56(1):115-122.
Murata K, Tsukuda S, Suizu F, Kimura A, Sugiyama M, Watashi K et al. 2020. Immunomodulatory mechanism of acyclic nucleoside phosphates in treatment of hepatitis b virus infection. Hepatology. 71(5):1533-1545.
Nakano T, Shapiro CN, Hadler SC, Casey JL, Mizokami M, Orito E et al. 2001. Characterization of hepatitis d virus genotype iii among yucpa indians in venezuela. J Gen Virol. 82(Pt 9):2183-2189.
Niro GA, Casey JL, Gravinese E, Garrubba M, Conoscitore P, Sagnelli E et al. 1999. Intrafamilial transmission of hepatitis delta virus: Molecular evidence. J Hepatol. 30(4):564-569.
Niro GA, Ciancio A, Tillman HL, Lagget M, Olivero A, Perri F et al. 2005. Lamivudine therapy in chronic delta hepatitis: A multicentre randomized-controlled pilot study. Aliment Pharmacol Ther. 22(3):227-232.
Niro GA, Smedile A, Ippolito AM, Ciancio A, Fontana R, Olivero A et al. 2010. Outcome of chronic delta hepatitis in italy: A long-term cohort study. J Hepatol. 53(5):834-840.
Nisini R, Paroli M, Accapezzato D, Bonino F, Rosina F, Santantonio T et al. 1997. Human cd4+ t-cell response to hepatitis delta virus: Identification of multiple epitopes and characterization of t-helper cytokine profiles. J Virol. 71(3):2241-2251.
Ocal S, Korkmaz M, Harmanci O, Ensaroglu F, Akdur A, Selcuk H et al. 2015. Hepatitis b- and hepatitis d-virus-related liver transplant: Single-center data. Exp Clin Transplant. 13 Suppl 1:133-138.
Ossami Saidy RR, Sud I, Eurich F, Aydin M, Postel MP, Dobrindt EM et al. 2021. Discontinuation of passive immunization is safe after liver transplantation for combined hbv/hdv infection. Viruses. 13(5).
Palom A, Rando-Segura A, Vico J, Pacin B, Vargas E, Barreira-Diaz A et al. 2022. Implementation of anti-hdv reflex testing among hbsag-positive individuals increases testing for hepatitis d. JHEP Rep. 4(10):100547.
Perez-Vargas J, Amirache F, Boson B, Mialon C, Freitas N, Sureau C et al. 2019. Enveloped viruses distinct from hbv induce dissemination of hepatitis d virus in vivo. Nat Commun. 10(1):2098.
Polaris Observatory C. 2023. Global prevalence, cascade of care, and prophylaxis coverage of hepatitis b in 2022: A modelling study. Lancet Gastroenterol Hepatol. 8(10):879-907.
Raimondo G, Brunetto MR, Pontisso P, Smedile A, Maina AM, Saitta C et al. 2006. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis b virus/hepatitis c virus-coinfected patients. Hepatology. 43(1):100-107.
Razavi HA, Buti M, Terrault NA, Zeuzem S, Yurdaydin C, Tanaka J et al. 2023. Hepatitis d double reflex testing of all hepatitis b carriers in low-hbv- and high-hbv/hdv-prevalence countries. J Hepatol. 79(2):576-580.
Rizzetto M. 1983. The delta agent. Hepatology. 3(5):729-737.
Rizzetto M. 2009. Hepatitis d: Thirty years after. J Hepatol. 50(5):1043-1050.
Romeo R, Del Ninno E, Rumi M, Russo A, Sangiovanni A, de Franchis R et al. 2009. A 28-year study of the course of hepatitis delta infection: A risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology. 136(5):1629-1638.
Romeo R, Foglieni B, Casazza G, Spreafico M, Colombo M, Prati D. 2014. High serum levels of hdv rna are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS One. 9(3):e92062.
Rosenau J, Kreutz T, Kujawa M, Bahr MJ, Rifai K, Hooman N et al. 2007. Hbsag level at time of liver transplantation determines hbsag decrease and anti-hbs increase and affects hbv DNA decrease during early immunoglobulin administration. J Hepatol. 46(4):635-644.
Sandmann L, Berg T, Deterding K, Fischer N, Hinrichsen H, Petersen J et al. 2023a. Antiviral therapy of chronic hepatitis d virus infection - addendum to the s3 guideline "prophylaxis, diagnosis and therapy of hepatitis b virus infection" of the german society for gastroenterology, digestive and metabolic diseases (dgvs). Z Gastroenterol. 61(12):e715-e732.
Sandmann L, Bremer B, Deterding K, Port K, Gey B, Fruchtel C et al. 2024a. Letter to the editor: The who hdv rna international standard does not reflect variability of real-world samples. Hepatology.
Sandmann L, Cornberg M. 2021. Experimental drugs for the treatment of hepatitis d. Journal of experimental pharmacology. 13:461-468.
Sandmann L, Degasperi E, Port K, Aleman S, Wallin JJ, Manuilov D et al. 2024b. Liver stiffness measurement as a noninvasive method for the diagnosis of liver cirrhosis in patients with chronic hepatitis d virus infection. Aliment Pharmacol Ther.
Sandmann L, Wedemeyer H. 2023b. Interferon-based treatment of chronic hepatitis d. Liver Int. 43 Suppl 1:69-79.
Sandmann L, Yurdaydin C, Deterding K, Heidrich B, Hardtke S, Lehmann P et al. 2022. Hbcrag levels are associated with virological response to treatment with interferon in patients with hepatitis delta. Hepatol Commun. 6(3):480-495.
Schaper M, Rodriguez-Frias F, Jardi R, Tabernero D, Homs M, Ruiz G et al. 2010. Quantitative longitudinal evaluations of hepatitis delta virus rna and hepatitis b virus DNA shows a dynamic, complex replicative profile in chronic hepatitis b and d. J Hepatol. 52(5):658-664.
Schirdewahn T, Grabowski J, Owusu Sekyere S, Bremer B, Wranke A, Lunemann S et al. 2017. The third signal cytokine interleukin 12 rather than immune checkpoint inhibitors contributes to the functional restoration of hepatitis d virus-specific t cells. J Infect Dis. 215(1):139-149.
Sheng WH, Hung CC, Kao JH, Chang SY, Chen MY, Hsieh SM et al. 2007. Impact of hepatitis d virus infection on the long-term outcomes of patients with hepatitis b virus and hiv coinfection in the era of highly active antiretroviral therapy: A matched cohort study. Clin Infect Dis. 44(7):988-995.
Smedile A, Casey JL, Cote PJ, Durazzo M, Lavezzo B, Purcell RH et al. 1998. Hepatitis d viremia following orthotopic liver transplantation involves a typical hdv virion with a hepatitis b surface antigen envelope. Hepatology. 27(6):1723-1729.
Soriano V, Grint D, d'Arminio Monforte A, Horban A, Leen C, Poveda E et al. 2011. Hepatitis delta in hiv-infected individuals in europe. AIDS. 25(16):1987-1992.
Soriano V, Vispo E, Sierra-Enguita R, Mendoza C, Fernandez-Montero JV, Labarga P et al. 2014. Efficacy of prolonged tenofovir therapy on hepatitis delta in hiv-infected patients. AIDS. 28(16):2389-2394.
Spaan M, Carey I, Bruce M, Shang D, Horner M, Dusheiko G et al. 2020. Hepatitis delta genotype 5 is associated with favourable disease outcome and better response to treatment compared to genotype 1. J Hepatol. 72(6):1097-1104.
Stern C, de Freitas C, Bazinet M, Mackiewicz V, Brichler S, Gordien E et al. 2023. Safety and efficacy of rep 2139-mg in association with tdf in chronic hepatitis delta patients with decompensated cirrhosis. Journal of Hepatology. 78(S1):1160.
Stockdale AJ, Kreuels B, Henrion MYR, Giorgi E, Kyomuhangi I, de Martel C et al. 2020. The global prevalence of hepatitis d virus infection: Systematic review and meta-analysis. J Hepatol. 73(3):523-532.
Su CW, Huang YH, Huo TI, Shih HH, Sheen IJ, Chen SW et al. 2006. Genotypes and viremia of hepatitis b and d viruses are associated with outcomes of chronic hepatitis d patients. Gastroenterology. 130(6):1625-1635.
Sureau C, Negro F. 2016. The hepatitis delta virus: Replication and pathogenesis. J Hepatol. 64(1 Suppl):S102-S116.
Takyar V, Surana P, Kleiner DE, Wilkins K, Hoofnagle JH, Liang TJ et al. 2017. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther. 45(1):127-138.
Taylor JM. 2012. Virology of hepatitis d virus. Semin Liver Dis. 32(3):195-200.
Terrault NA, Lok ASF, McMahon BJ, Chang K-M, Hwang JP, Jonas MM et al. 2018. Update on prevention, diagnosis, and treatment of chronic hepatitis b: Aasld 2018 hepatitis b guidance. Hepatology 67(4):1560-1599.
Tsatsralt-Od B, Takahashi M, Endo K, Buyankhuu O, Baatarkhuu O, Nishizawa T et al. 2006. Infection with hepatitis a, b, c, and delta viruses among patients with acute hepatitis in mongolia. J Med Virol. 78(5):542-550.
Tsatsralt-Od B, Takahashi M, Nishizawa T, Endo K, Inoue J, Okamoto H. 2005. High prevalence of dual or triple infection of hepatitis b, c, and delta viruses among patients with chronic liver disease in mongolia. J Med Virol. 77(4):491-499.
Wedemeyer H. 2010a. Re-emerging interest in hepatitis delta: New insights into the dynamic interplay between hbv and hdv. J Hepatol. 52(5):627-629.
Wedemeyer H. 2020. The burden of hepatitis d - defogging the epidemiological horizon. J Hepatol. 73(3):493-495.
Wedemeyer H, Aleman S, Brunetto M, Blank A, Andreone P, Bogomolov P et al. 2024. Bulevirtide monotherapy in patients with chronic hdv: Efficacy and safety results through week 96 from a phase iii randomized trial. J Hepatol. 81(4):621-629.
Wedemeyer H, Aleman S, Brunetto MR, Blank A, Andreone P, Bogomolov P et al. 2023a. A phase 3, randomized trial of bulevirtide in chronic hepatitis d. N Engl J Med. 389(1):22-32.
Wedemeyer H, Leus M, Battersby TR, Glenn J, Gordien E, Kamili S et al. 2023b. Hdv rna assays: Performance characteristics, clinical utility, and challenges. Hepatology.
Wedemeyer H, Manns MP. 2010b. Epidemiology, pathogenesis and management of hepatitis d: Update and challenges ahead. Nature reviews Gastroenterology & hepatology. 7(1):31-40.
Wedemeyer H, Schoneweis K, Bogomolov P, Blank A, Voronkova N, Stepanova T et al. 2023c. Safety and efficacy of bulevirtide in combination with tenofovir disoproxil fumarate in patients with hepatitis b virus and hepatitis d virus coinfection (myr202): A multicentre, randomised, parallel-group, open-label, phase 2 trial. Lancet Infect Dis. 23(1):117-129.
Wedemeyer H, Schöneweis K, Bogomolov PO, Voronkova N, Chulanov V, Stepanova T et al. 2019a. Gs-13-final results of a multicenter, open-label phase 2 clinical trial (myr203) to assess safety and efficacy of myrcludex b in cwith peg-interferon alpha 2a in patients with chronic hbv/hdv co-infection. Journal of Hepatology. 70(1):e81.
Wedemeyer H, Yurdaydin C, Dalekos GN, Erhardt A, Cakaloglu Y, Degertekin H et al. 2011. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med. 364(4):322-331.
Wedemeyer H, Yurdaydin C, Hardtke S, Caruntu FA, Curescu MG, Yalcin K et al. 2019b. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis d (hidit-ii): A randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 19(3):275-286.
Williams V, Brichler S, Radjef N, Lebon P, Goffard A, Hober D et al. 2009. Hepatitis delta virus proteins repress hepatitis b virus enhancers and activate the alpha/beta interferon-inducible mxa gene. J Gen Virol. 90(Pt 11):2759-2767.
Wranke A, Hardtke S, Heidrich B, Dalekos G, Yalcin K, Tabak F et al. 2020. Ten-year follow-up of a randomized controlled clinical trial in chronic hepatitis delta. J Viral Hepat. 27(12):1359-1368.
Wranke A, Heidrich B, Deterding K, Hupa-Breier KL, Kirschner J, Bremer B et al. 2023. Clinical long-term outcome of hepatitis d compared to hepatitis b monoinfection. Hepatol Int. 17(6):1359-1367.
Wranke A, Lobato C, Ceausu E, Dalekos GN, Rizzetto M, Turcanu A et al. 2024. Long-term outcome of hepatitis delta in different regions world-wide: Results of the hepatitis delta international network. Liver Int. 44(9):2442-2457.
Wranke A, Serrano BC, Heidrich B, Kirschner J, Bremer B, Lehmann P et al. 2017. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology. 65(2):414-425.
Yurdaydin C, Bozkaya H, Gurel S, Tillmann HL, Aslan N, Okcu-Heper A et al. 2002. Famciclovir treatment of chronic delta hepatitis. J Hepatol. 37(2):266-271.
Yurdaydin C, Keskin O, Kalkan C, Karakaya F, Caliskan A, Kabacam G et al. 2018a. Interferon treatment duration in patients with chronic delta hepatitis and its effect on the natural course of the disease. J Infect Dis. 217(8):1184-1192.
Yurdaydin C, Keskin O, Kalkan C, Karakaya F, Caliskan A, Karatayli E et al. 2018b. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The lowr hdv-1 study. Hepatology. 67(4):1224-1236.
Zachou K, Yurdaydin C, Drebber U, Dalekos GN, Erhardt A, Cakaloglu Y et al. 2010. Quantitative hbsag and hdv-rna levels in chronic delta hepatitis. Liver Int. 30(3):430-437.
Zhang Z, Ni Y, Lempp FA, Walter L, Mutz P, Bartenschlager R et al. 2022. Hepatitis d virus-induced interferon response and administered interferons control cell division-mediated virus spread. J Hepatol. 77(4):957-966.
Zollner C, Hofmann J, Lutz K, Tacke F, Demir M. 2022. Real-life experiences with bulevirtide for the treatment of hepatitis delta-48 weeks data from a german centre. Liver Int. 42(11):2403-2407.
