2. Hepatitis B – treatment
Heinrich Rodemerk, Thomas Berg, Florian van Bömmel
Introduction
Treatment of Hepatitis B is a complex and dynamic field. Since the approval of the first interferon-based treatment for Hepatitis B in the late 20th century, new antiviral substances, such as nucleos(t)ide analogues have been introduced and further developed. An even wider range of possible new therapeutic options is currently being investigated in studies.
A subgroup of patients with a chronic HBV infection progresses to chronic Hepatitis B (CHB). Those patients carry an elevated risk for liver-related mortality and morbidity. Identifying the patients that benefit most from antiviral therapy is crucial for reducing the risk of fibrosis, cirrhosis, decompensation and hepatocellular carcinoma (HCC) development. Several factors influence the choice of the optimal treatment out of available options. Regular monitoring of patient-related and viral factors should accompany any therapeutic action. Although a sterile and complete cure for Hepatitis B is not yet possible, different therapeutic endpoints can be reached with current treatment. Novel approaches, such as treatment cessation after long-term application of nucleos(t)ide analogues or combination of new substances may induce functional cure.
Before commencing any form of treatment, some main questions need to be considered:
- Why treat?
- Who to treat?
- How to treat?
- How to monitor treatment?
- When to stop?
This chapter aims to provide an overview of therapeutic options and may help to answer some of the questions above. However, an individualised and patient-centred approach should be maintained and all relevant factors in the clinical situation need to be considered. Hepatitis B care, including antiviral treatment, should be delivered according to regularly updated guidelines. There are different regional and international guidelines reflecting the current state of the art for Hepatitis B care (see Table 1). Several context factors can influence the clinical decision, so other guidelines may be relevant in different parts of the world, even if not listed here.
Table 1. Guideline overview| Institution | Year | Full Name | Reference |
| World Health Organization (WHO) | 2024 | Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection | (WHO 2024) |
| European Association for the Study of the Liver (EASL) | 2017* | EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection | (EASL 2017) |
| American Association for the Study of Liver Diseases (AASLD) | 2018 | Update on Prevention, Diagnosis, and Treatment and of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance | (Terrault 2018) |
| Asian Pacific Association for the Study of the Liver (APASL) | 2016 | Asian-Pacific clinical practice guidelines on the management of hepatitis B | (Sarin 2016) |
| The Korean Association for the Study of the Liver (KASL) | 2022 | KASL clinical practice guidelines for management of chronic hepatitis B | (KASL 2022) |
| Turkish Association for the Study of the Liver (TASL) | 2017 | Diagnosis, management and treatment of hepatitis B virus infection: Turkey 2017 Clinical Practice Guidelines | (Tabak 2017) |
| German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS) | 2021 | S3 Guideline of the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS) on the Prophylaxis, Diagnosis, and Treatment of Hepatitis B Virus Infection | (Cornberg 2021) |
Treatment goals (Why treat?)
Hepatitis B is still a major public health threat. The overall rationale for Hepatitis B testing, treatment and care is to lower the disease burden on a population level. Besides public health approaches that mainly focus on prevention, current treatment strategies are designed to reach therapeutic endpoints that reduce the individual risk for liver-related mortality and morbidity. To date, the full eradication of HBV (sterile cure) is impossible to achieve by available treatment options. This is due to the persistence of episomal covalently closed circular DNA (cccDNA), a template of the HBV genome located in the nucleus of infected hepatocytes (Rehermann 1996). Thus, the main treatment goal is to improve the patient’s survival and quality of life by preventing disease progression, hepatocyte and parenchyma damage, complications and consequently HCC development. Reducing the risk of HBV transmission is an additional goal of antiviral therapy (EASL 2017; Terrault 2018; WHO 2024). Reactivation of an HBV infection may occur in certain circumstances from the nuclear reservoirs even decades after HBsAg loss. Prophylactic antiviral therapy should be used in patients undergoing (induced) immunosuppression to prevent reactivation. In patients with acute Hepatitis B, preventing the risk of acute liver failure is the main treatment goal.
Future therapeutic options aim to cure CHB by eliminating all replicative forms of HBV. The ultimate goal is the global elimination of HBV infection by various strategies, including vaccination, treatment and prevention of transmission (Sarin 2016).
Forms of cure and therapeutic endpoints
Different categories of “cure” have been defined, and they serve as endpoints that should be reached by CHB treatment:
- Virological cure: Suppression of HBV DNA to undetectable levels
- HBeAg loss: seroconversion from detectable HBeAg to anti-HBe
- Functional cure: HBsAg loss +/- seroconversion to anti-HBs
- Partial functional cure: inactive carrier state with low levels of HBsAg and HBV DNA, off-treatment
- Sterile cure: no form of HBV-DNA detected, including integrated forms and cccDNA
Virological cure refers to the suppression of the HBV replication to undetectable levels. It is one major goal in treatment. The continuous suppression of serum HBV DNA over several years shows a time-dependent reversion of liver fibrosis as well as a decrease in the HCC risk. The regression of liver fibrosis during antiviral treatment was impressively demonstrated in a subanalysis of two trials with patients who underwent biopsies before and after five years of TDF monotherapy (Marcellin 2013). 88% of the patients experienced an improvement in overall liver histology. Of patients who had cirrhosis at the start of therapy, 73% experienced regression of cirrhosis, and 72% had at least a two-point reduction in fibrosis scoring. The positive effect of antiviral treatment on liver histology was also shown in a subgroup of patients from a rollover study including two phase III trials on the efficacy of ETV in treatment-naïve patients. Liver biopsies taken at baseline and after a median treatment duration of 6 years showed a substantial histologic improvement in 96% of the patients (Chang 2010b). Ongoing viral replication is a key risk factor for HCC development. Antiviral treatment reduces that risk by 30% (in cirrhosis) up to 80% (in non-cirrhosis), as first shown for Asian cohorts (Papatheodoridis 2015). The decrease in HCC incidence during antiviral treatment was illustrated by the results of a retrospective analysis comparing HBV-infected Taiwanese either being treated with antivirals or not. Among the patients receiving treatment with NAs, the incidence rate of HCCs over 7 years of follow-up was 7.3 % compared to 22.9% in patients without antiviral treatment (Wu 2014). However, the HCC risk is not affected immediately after the initiation of antiviral treatment. Thus, the incidence of HCCs was shown to start decreasing after 5 years of effective HBV DNA suppression by either Entecavir or Tenofovir (Papatheodoridis 2017). After eight years of treatment, it was similar to individuals without HBV infection in a multicentric European cohort (Papatheodoridis 2018). The presence of liver cirrhosis strongly determines the remaining HCC risk. However, also patients with liver cirrhosis show a decreasing incidence of HCC development following treatment (Su 2016). Overall, these data indicate that with potent NAs, the HCC risk can be reduced but not eliminated.
HBeAg loss. HBeAg seroconversion is another treatment endpoint, as long as HBV replication remains durably suppressed to low levels. In HBeAg-positive patients, seroconversion from HBeAg to anti-HBe was found to be a reliable surrogate marker for prognosis of chronic HBV infection leading in many cases to an inactive HBsAg carrier state. In these patients, HBsAg remains detectable but HBV replication continues at low or even undetectable levels and transaminases are generally within normal ranges. HBeAg seroconversions that appear during antiviral treatment can be considered a lasting immune response in the majority of patients. In a meta-analysis, in 76% of patients, the HBeAg seroconversion was stable after treatment discontinuation (Papatheodoridis 2016b). However, long-term observations reveal that HBeAg seroconversion cannot always be taken as a guarantee of long-term remission. A reactivation of the disease with “sero-reversion” (HBeAg becoming detectable again) as well as a transition to HBeAg-negative CHB with increased and often fluctuating HBV DNA levels may occur in 30-50% of patients (Hadziyannis 2001; Hadziyannis 2006a; van Hees 2018). Therefore, HBeAg seroconversion should only be regarded as a treatment endpoint in conjunction with durable and complete suppression of HBV replication. There is an ongoing discussion about whether and how long a consolidation treatment (6-12 months) should be maintained following HBeAg seroconversion. As a result, Asian guidelines recommend stopping treatment immediately after HBeAg seroconversion, whereas American and European guidelines favour treatment continuation, but allow discontinuation in selected patients with close subsequent monitoring.
HBsAg loss. Since HBsAg loss or seroconversion is associated with a complete and definitive remission of disease activity and an improved long-term outcome, it is currently regarded as a “functional cure” and a stable remission of HBV infection, although HBV cccDNA persists in infected hepatocytes and reactivations may occur. Unfortunately, HBsAg loss can be induced in only a limited number of patients by treatment (in up to 10% of HBeAg-positives and in <1% of HBeAg-negatives) (Moini 2022). The probability of HBsAg seroclearance during therapy with NAs is linked to a decrease in HBsAg levels during the early treatment period. As HBsAg levels remain unchanged in most patients during the first years of treatment it seems therefore unlikely that a longer duration of NA treatment will further increase rates of HBsAg loss (Marcellin 2011). Due to a greatly reduced risk in most hepatic outcomes on morbidity and mortality, HBsAg loss can be regarded as the most important endpoint (Morais 2023).
Partial functional cure/Sustained immune control. The term “sustained immune control” can be used to describe a stage that follows the discontinuation of treatment for Hepatitis B, either in NA- or PegIFN-based treatments. It describes the “absence of virological treatment indication” and refers to a stage with low HBV replication (ideally < 2.000 IU/mL) and normal ALT levels but detectable HBsAg (and possibly HBeAg). However, the durability of this immune control is not guaranteed due to the fluctuating course of HBeAg-negative CHB. For treatment with PEG-IFN α in both HBeAg-positive and -negative patients, inducing an immune control status, characterised by persistent suppression of viral replication with HBV DNA levels below 2, 000 IU/mL and normalisation of ALT levels was defined as a treatment endpoint (Marcellin 2009). If this condition is maintained over time, it increases the probability of HBsAg loss and reduces the development of liver fibrosis and HCC. Late relapse beyond 6 months post-treatment has been described, but a sustained response at one year post-treatment appears to be durable through long-term follow-up (Marcellin 2009). However, the immune control status needs to be regularly monitored, and treatment has to be reintroduced in cases with an increased HBV replication. Immune control defined as the “absence of treatment indication” was recently shown to be an important endpoint after discontinuation of long-term antiviral treatment in HBeAg-negative patients (Berg 2017). For patients presenting any signs of liver fibrosis or a family history of HCC, immune control should not be regarded as a treatment endpoint but rather the complete suppression of HBV replication.
Sterile cure: This term refers to the complete absence of HBV DNA and its integrates in hepatocytes. With currently used antivirals this endpoint is not achievable. In difference to functional cure with loss of HBsAg, there is no suspected risk of reactivation. Patients would have a similar HBV-attributable liver-related mortality as individuals who have never been infected.
Indication for antiviral therapy (Who to treat?)
Acute hepatitis B
Acute Hepatitis B resolves spontaneously in 95-99% of cases (McMahon 1985; Tassopoulos 1987; EASL 2017). Therefore, treatment of acute HBV infections with the currently available drugs is generally not indicated. In a study from India, treatment with LAM in patients with acute Hepatitis B showed no significantly greater biochemical and clinical improvement compared to placebo (Kumar 2007). However, in patients with a potentially life-threatening disease course as severe or fulminant acute Hepatitis B, antiviral treatment should be at least considered. There are observations suggesting that antiviral treatment might reduce mortality in patients experiencing fulminant hepatitis during acute HBV infection. Thus, in a trial comparing treatment with LAM 100mg once daily versus no treatment in Chinese patients with fulminant Hepatitis B, a mortality of 7.5% was found in patients receiving LAM treatment compared to 25% in the control group. The earlier the treatment was initiated, the better the results obtained (Yu 2010). Several case reports from Europe also indicate that patients with severe and fulminant Hepatitis B may benefit from early antiviral therapy with LAM or other NAs by reducing the need for high-urgency liver transplantation (Tillmann 2006). NAs appear to be safe in patients with fulminant Hepatitis B and do not increase the risk for chronification (Jochum 2016). As a result, antiviral treatment of fulminant or severe acute Hepatitis B with NAs is recommended by current treatment guidelines (Sarin 2016; EASL 2017; Terrault 2018; WHO 2024). Interferon therapy is generally not recommended in patients with acute HBV infection due to the risk of liver failure by increasing the inflammatory activity. The endpoint of treatment of acute HBV infections is HBsAg clearance (Su 2016; EASL 2017).
Chronic hepatitis B
Due to the large interindividual differences in the natural course of HBV infection, it is necessary to identify patients with a higher risk for HBV-related mortality. Those patients benefit from specific antiviral therapy. All individuals with HBV viraemia should initially be considered as potential candidates for antiviral therapy due to the oncogenic potential of HBV (Chen 2006; Iloeje 2006; EASL 2017; Terrault 2018). However, a new nomenclature was introduced to distinguish patients with ongoing inflammation and a higher risk from those with a less active form of infection. Most guidelines base their recommendations on who to treat on this differentiation. While patients with signs of active chronic Hepatitis B, defined by high viraemia, increased transaminases and/or (non-invasive) indicators of tissue damage should usually be treated, patients with chronic HBV infection are usually subject to regular monitoring. Table 2 shows the main differences between chronic Hepatitis B and chronic HBV infection.
Table 2. Hepatitis B nomenclature| HBeAg-positive | HBeAg-negative | |||
| Chronic infection | Chronic hepatitis | Chronic infection | Chronic hepatitis | |
| HBsAg | High | High/Intermediate | Low | Intermediate |
| HBeAg | Positive | Positive | Negative | Negative |
| HBV DNA | ≥ 107 IU/mL | 104-107 IU/mL | < 2, 000 IU/mL* | ≥ 2, 000 IU/mL |
| ALT | Normal | Elevated | Normal | Elevated** |
| Liver disease | None/minimal | Moderate/severe | None | Moderate/severe |
| Old terminology | Immune tolerant | Immune reactive HBeAg positive | Inactive carrier | HBeAg negative chronic hepatitis |
**Persistently or intermittently. Adapted from: (EASL 2017)
There is widespread agreement that the decision on whether to initiate treatment should be made on the following criteria (Sarin 2016; EASL 2017; Tabak 2017; Terrault 2018; Cornberg 2021; KASL 2022; WHO 2024):
- serum HBV-DNA levels,
- ALT elevation
- histologic changes in liver tissue
In Table 3, the key recommendations for treatment initiation from different guidelines are listed. It is important to note, that the defined upper limit of normal (ULN) of alanine aminotransferase (ALT) levels varies geographically, therefore different guidelines have set different cut-offs. The WHO guidelines define a cut-off at 30 IU/L for men and boys and 19 IU/L for women and girls, almost similar to the AASLD guidelines, whereas the KASL guidelines define 34 IU/L for males and 30 IU/L for females as ULN. In the EASL and APASL guidelines, 40 IU/L is set as ULN for both sexes.
Besides the assessment of inflammation, indication for treatment should also take into account age, health status, family history of HCC or cirrhosis and extrahepatic manifestations. The HBeAg-status is not necessary anymore for treatment indication, although concerning the choice of the appropriate antiviral drug (NAs vs. Interferon α), this criterium may still be useful.
Table 3. Recommendation upon treatment initiation| Guideline | Treat all HBsAg-positive patients with: |
| WHO |
|
| EASL |
|
| AASLD |
|
| APASL |
|
| KASL |
|
| TASL |
|
| DGVS |
|
While treatment recommendations vary slightly among the different guidelines, in the majority of them, the most important factor for a decision to initiate treatment has shifted from histologically proven disease activity to the serum levels of HBV DNA. Thus, most guidelines recommend antiviral treatment for patients with HBV DNA levels >2, 000 IU/mL (corresponding to >10, 000 copies/mL) in association with a sign of ongoing hepatitis (elevated ALT levels) or liver fibrosis demonstrated by liver histology greater than A1/F1. If available, non-invasive tools such as liver elastography or serologic algorithms should be used, especially if patients are reluctant to have a liver biopsy (EASL 2017; WHO 2024). The treatment algorithm from the EASL guidelines is displayed in Figure 1.
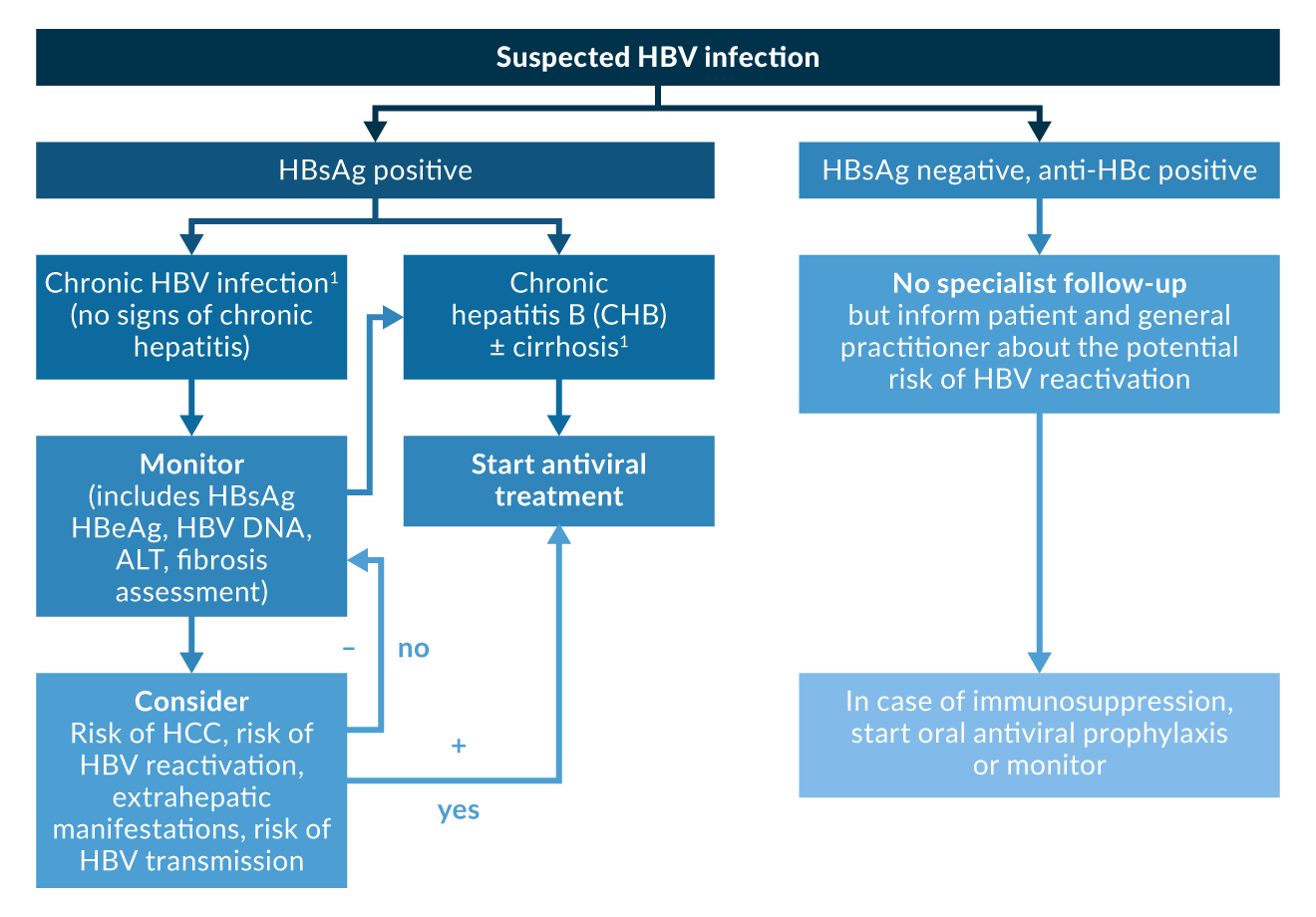 Figure 1. Hepatitis B treatment algorithm from EASL Clinical Practices Guidelines (EASL 2017).
Figure 1. Hepatitis B treatment algorithm from EASL Clinical Practices Guidelines (EASL 2017).
Treatment of HBV infections in special populations
Cirrhosis
In patients with liver cirrhosis and detectable HBV DNA, treatment is recommended in most guidelines, regardless of serum HBV DNA levels or ALT elevation (EASL 2017; Cornberg 2021; KASL 2022; WHO 2024). Other guidelines include a strong recommendation for antiviral treatment only in decompensated cirrhosis but suggest considering treatment in compensated cirrhosis with low-level viraemia (Terrault 2018). In patients with decompensated cirrhosis with Child-Pugh-Score B or C, standard or pegylated Interferon-α is contraindicated.
HBeAg-negative HBV infection
It is yet under debate if there is a benefit in treating all patients with detectable viraemia, even without signs of hepatitis. In HBeAg-negative HBV infection (former “inactive HBsAg carriers”) characterised by positive anti-HBe, HBV DNA levels below 2, 000 IU/mL and serum aminotransferases within normal ranges, therapy is currently not recommended by most guidelines (Sarin 2016; EASL 2017; Terrault 2018; Cornberg 2021). The risk of liver-related mortality in patients without biochemical or histological signs of hepatitis or parenchyma damage was not elevated in European HBsAg carriers compared to uninfected individuals (Manno 2004). The current WHO guideline regards low viraemia as only one factor to be considered. Treatment is also recommended in HbsAg-positive patients with any form of fibrosis or any of the following co-factors: coinfection (e.g. HIV, HDV, HCV), family history of liver cancer or cirrhosis, immune suppression, comorbidities (e.g. diabetes, metabolic dysfunction-associated steatotic liver disease) or extrahepatic manifestations (e.g. glomerulonephritis or vasculitis). If quantitative HBV DNA assays are unavailable, any ALT above ULN is seen as a treatment indication (WHO 2024). See Figure 2 for the updated WHO treatment algorithm. These recommendations apply to a much broader range of patients. In addition, the REVEAL study demonstrated, that patients with HBeAg-negative HBV infection still had a substantial risk for HCC (Chen 2010).
The differentiation between true inactive chronic HBV infection and patients with chronic HBeAg-negative hepatitis may be difficult in some cases. Elevated transaminases are no reliable parameter for assessing the stage of liver fibrosis and long-term prognosis of HBV-infected individuals. Even in patients with normal or only slightly elevated aminotransferases, there can be a significant risk for the development of HBV-associated complications (Chen 2006; Iloeje 2006; Chen 2010). HBsAg levels are useful for predicting the risk of HBV reactivation with subsequent replication and inflammatory activity (Martinot-Peignoux 2013; Tseng 2013). Newer biomarkers, such as quantitative HBV RNA may help to distinguish patients with a true inactive HBV infection from those with a higher risk for reactivation (Testoni 2024). Antiviral treatment reduces the risk of HBV-related mortality if used in early phases with high viraemia, but does not affect endpoints when serum HBV-DNA levels are low (Huang 2023; Choi 2024). Furthermore, antiviral treatment can’t fully eliminate the risk of HCC. Therefore, the benefits of antiviral therapy must be carefully weighed against the higher off-treatment chance of spontaneous HBsAg loss and the relevant side effects of long-term NA treatment (Yip 2024).
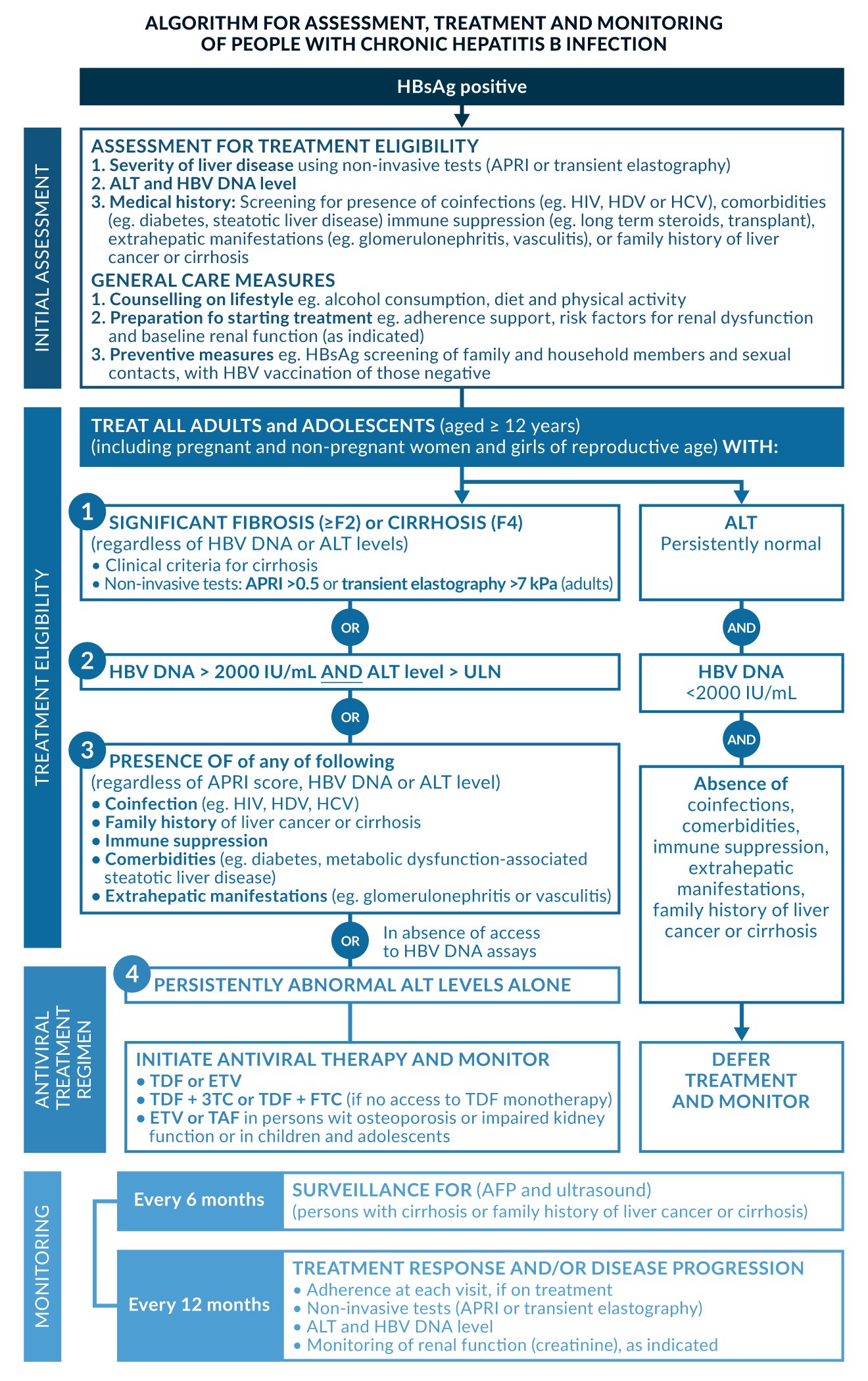 Figure 2. Algorithm for assessment, treatment and monitoring of people with chronic Hepatitis B infection, reproduced from WHO guidelines. ALT ULN: male: 30 IU/L, female: 19 IU/L (WHO 2024).
Figure 2. Algorithm for assessment, treatment and monitoring of people with chronic Hepatitis B infection, reproduced from WHO guidelines. ALT ULN: male: 30 IU/L, female: 19 IU/L (WHO 2024).
Pregnancy
Globally, vertical transmission from the mother to the newborn is the most frequent cause of HBV infection. The highest risk occurs during delivery, especially if the maternal viraemia is high in HBeAg-positive Hepatitis B. To prevent transmission, guidelines recommend the active Hepatitis B vaccination of the newborn infant as soon as possible, preferably within the first 12-24 hours (WHO 2024), followed by 2 to 3 additional doses in a routine scheme. A combination of Hepatitis B immunoglobulin may further reduce the risk of transmission to less than 5% (Veronese 2021). Still, for a neonate born to a mother with high levels of HBV DNA (over 200, 000 IU/mL), the risk of perinatal transmission is considerable. Therefore, antiviral treatment is generally recommended in these women (EASL 2017; Terrault 2018; Cornberg 2021; WHO 2024). PEG-IFN α is contraindicated. In pregnant women with high levels of HBV DNA, LAM treatment during the last trimester of pregnancy was reported to reduce the risk of intrauterine and perinatal transmission of HBV if given in addition to passive and active vaccination (van Zonneveld 2003). Due to its high antiviral potency, TDF is often considered the treatment of choice. The risk of teratogenicity of NAs is assessed by a classification based on data gathered in clinical trials as well as through the FDA Pregnancy Registry. TDF and LAM are listed as pregnancy category B drugs, whereas ADV and ETV are category C drugs. However, side effects on the newborn cannot completely be ruled out. A recent study reported that bone mineral content in infants of HIV-infected mothers exposed to TDF was 12% lower than in non-exposed (Siberry 2015). In a comparative study, LdT, TDF and TAF were similarly very effective in preventing mother-to-child transmission. However, in the TAF group, a higher amount of cardiac abnormalities was observed (Pan 2024b). The benefits of maternal treatment in preventing mother-to-child transmission must be carefully weighed against potential risks for maternal and infant health. A recent meta-analysis found no relevant safety concerns in NA treatment (Pan 2024a). As exacerbations of the HBV infection may occur, women with HBV should be monitored closely after delivery (Borg 2008).
Immunosuppression
During immunosuppressive treatment, an asymptomatic or inactive HBV infection may reactivate in 20% to 50% of patients (Lau 2021). These reactivations can occur in both inactive chronic HBV infections and in patients with functional cures (HBsAg-negative, but anti-HBc-positive patients). They are characterised by an increase in HBV replication followed by signs of liver inflammation during immune reconstitution resulting in liver damage or even liver failure in some patients (Artz 2010; Roche 2011). Immunosuppressive therapies with the highest risk of HBV reactivation are chemotherapeutic treatment for cancer and advanced anti-autoimmune and antirheumatic treatment. This includes anti-CD20 therapies (rituximab), treatment with corticosteroids and TNF-α inhibitors (i.e. infliximab, etanercept, adalimumab), tyrosine kinase inhibitors (i.e. imatinib) or other biologicals (i.e. abatacept, anakinra, tocilizumab) and stem cell transplantation. Some cases of HBV reactivation have also been observed in other forms of immunosuppression, such as trans-arterial chemoembolisation for HCC or immunosuppressive therapy after solid organ transplantation (Moses 2006; Vassilopoulos 2007; Lau 2021). Prior to initiating immunosuppressive therapies, screening for HBV infection is recommended (EASL 2017; Lau 2021). Pre-emptive therapy should be considered for:
- all patients with active Hepatitis B before any immunosuppressive treatment
- HBsAg-positive chronic HBV infections receiving moderate to aggressive immunosuppression, depending on the individual risk
- anti-Hbc-positive, HBsAg-negative patients when therapy with a high risk of reactivation is planned (i.e. rituximab or human stem cell transplantation)
If available, highly potent antivirals, such as ETV or TDF, should be used for pre-emptive treatment. Termination of antiviral therapy can be considered 6 months after the end of immunosuppression (Lau 2021).
Treatment options and choice (How to treat?)
Currently, there are two main options for medical treatment of CHB: pegylated Interferon (PEG-IFN) or nucleoside/nucleotide analogues (NAs). The option of PEG-INF α-treatment may be considered for all patients in the first line, however, there are many contraindications, making them unsuitable for several subgroups of CHB patients. In contrast, NAs can be used in almost all clinical situations. Factors influencing the decision on which drug to use will be discussed under the subheading “Choosing the right treatment option”.
Interferons
INF α is a naturally occurring cytokine with immune modulatory, antiproliferative and antiviral activity. During treatment, the therapeutic efficacy of INF α can often be clinically recognised by a self-limited increase of ALT levels to at least twice the baseline levels. These ALT flares are frequently associated with virologic response. The main goal of INF α treatment is to induce long-term remission after a finite treatment duration. Response to IFN α can be either HBeAg seroconversion or durable suppression of HBV DNA to low or undetectable levels. In these responders, the chance for HBsAg loss in the long-term is relatively high.
Table 4. Interferon overview| Treatment Option | Dosage | Advantage/disadvantage |
| Standard INF α | 5-10 Mio. IU 3x/week | + first approved CHB treatment |
| – subcutaneous injection every other day | ||
| PEG-INF α | 180 µg/week | + application once weekly + high rates of HBe seroconversion + high rates of sustained virological suppression after termination + high rates of sustained virological suppression after termination |
| – many side effects – a considerable amount of non-responders – not useful in certain clinical situations (cirrhosis, prophylaxis, pregnancy) |
Standard INF α. Standard IFN α was approved for the treatment of CHB in 1992. IFN α is applied in dosages ranging from 5 million units (MU) to 10 MU every other day or thrice weekly. In a meta-analysis, a significant improvement in endpoints was shown in patients with HBeAg-positive chronic Hepatitis B being treated with standard IFN compared to untreated patients (Craxì 2003). Complete remission of fibrotic changes was observed in some patients and the loss of HBsAg occurred comparatively often. Furthermore, there was a trend towards less hepatic decompensation (treated 8.9% vs. untreated 13.3%), hepatocellular carcinoma (1.9% vs. 3.2%), and liver-associated deaths (4.9% vs. 8.7%) (Craxì 2003). A significant decrease in ALT and HBV DNA serum levels was also shown for standard IFN α in the treatment of HBeAg-negative CHB (Brunetto 2003). However, a high percentage of these patients relapse after the end of treatment showing elevation of ALT levels and a return of HBV DNA levels. The relapse rate seems to be higher when treatment duration is short (16 to 24 weeks) compared to longer treatment (12 to 24 months). A retrospective comparison of IFN therapies lasting from 5 to 12 months showed, that prolonged treatment increased the chance of a long-term response, concerning ALT normalisation and HBV DNA suppression. The overall response rates were 54% at the end of therapy, 24% at 1 year after therapy, and 18% 7 years after therapy (Manesis 2001). Patients with long-term response to treatment have a more favourable outcome for progression to liver cirrhosis, liver-associated deaths and development of hepatocellular carcinoma than patients who were untreated, unresponsive, or had a relapse (Brunetto 2003; Lampertico 2003). However, due to higher antiviral efficacy, PEG-IFN α should be preferred to standard IFN α.
PEG-INF α. The addition of a polyethylene glycol molecule to the interferon resulted in a significant increase in half-life, thereby allowing administration once weekly. Two types of subcutaneously administered PEG-IFN α were developed: PEG-IFN α-2a and PEG-IFN α-2b. PEG-IFN α-2a was licensed for the treatment of chronic HBV infections in a weekly dose of 180 µg for 48 weeks in both HBeAg-positive and HBeAg-negative patients. Both forms show similar efficacy. After one year of treatment with PEG-IFN α-2a and α-2b, 22% to 27% of patients were reported to achieve HBeAg seroconversion (Janssen 2005; Lau 2005) The safety profiles of standard IFN α and PEG-IFN α are similar. After termination of therapy, a relatively high relapse rate can be expected (>50%). The dose of 180 µg per week applied for 48 weeks was shown to exert a stronger antiviral efficacy compared to administration for 24 weeks or to the administration of 90 µg per week (Manesis 2001; Liaw 2011). Treatment for longer than 48 weeks is not recommended in current guidelines.
PEG-IFN α in HBeAg-positive patients. Several randomised, controlled studies investigating the efficacy of PEG-IFN α in HBeAg-positive patients have been conducted (Chan 2005; Janssen 2005; Lau 2005). These studies compared 180 µg PEG-INF α per week to standard IFN, LAM, and/or combination treatment with PEG-INF α + LAM for 48 weeks. Sustained HBeAg seroconversion at the end of follow-up (week 72) was significantly higher in patients treated with PEG-IFN α-2a alone or in combination with LAM than in patients treated with LAM alone (32% and 27% versus 19%) (Marcellin 2004).
PEG-IFN α in HBeAg-negative patients. The efficacy and safety of 48 weeks of treatment with 180 µg PEG-IFN α-2a once weekly, with LAM 100 mg daily and the combination of LAM and PEG-IFN α-2a was compared in HBeAg-negative patients. After 24 weeks of follow-up, the percentage of patients with normalisation of ALT levels or HBV DNA levels below 20, 000 copies/mL was significantly higher with PEG-IFN α-2a monotherapy and a combination of PEG-IFN α-2a plus LAM than with LAM monotherapy. The rates of sustained suppression of HBV DNA below 400 copies/mL were 19% with PEG-IFN α-2a monotherapy, 20% with combination therapy, and 7% with LAM alone (Lau 2005). Prolongation of PEG-IFN α treatment beyond 48 weeks may increase sustained response rates in HBeAg-negative patients. This was found in an Italian study with HBeAg-negative patients who were randomised to either treatment with 180 µg PEG-IFN α-2a per week for 48 weeks or additional treatment with PEG-IFN α-2a 135µg per week for another 48 weeks. As a result, 48 weeks after the end of treatment, 26% of patients who had received a longer treatment course showed HBV DNA suppression below 2, 000 IU/mL as compared to only 12% of the patients who had received PEG-IFN α-2a for 48 weeks only. Combination with LAM showed no additional effect (Lampertico 2013). However, the prediction of response and management of side effects during prolonged treatment with PEG-IFN α has not yet been established and it is not recommended for clinical practice. Importantly, it was shown that PEG-IFN α obviously induces immune modulatory effects which lead to considerable HBsAg clearance rates during the long–term follow-up period after treatment termination. In a study, HBeAg-positive patients with chronic HBV infection who had received treatment with standard IFN α were retrospectively analysed for a median period of 14 years. During the observation period, almost a third of this cohort lost HBsAg (Moucari 2009).
Nucleoside and nucleotide analogues
NAs inhibit HBV replication by competing with the natural substrate deoxyadenosine triphosphate (dATP) and therefore causing termination of the HBV DNA chain prolongation. They represent two different subclasses of reverse transcriptase inhibitors: while both are based on purines or pyrimidines, acyclic nucleotide analogues have an open (acyclic) ribose ring that confers greater binding capacity to resistant HBV polymerase strains. The optimal treatment duration for NAs is not yet defined, but treatment cessation after application of these agents for 48 weeks is associated with prompt relapse in viraemia, so they should be administered for longer periods. The treatment efficacy of NAs is defined by a complete suppression of HBV DNA levels in serum. This should be achieved within at least 6-12 months if agents with moderate to high risk for resistance development, such as LAM, ADV, and LdT, are used. Cumulative data concerning resistance rates in NAs are displayed in Figure 3. Effective and durable control of HBV replication with NAs is associated with a reduction of long-term complications such as liver cirrhosis and the development of HCC, especially in patients with liver cirrhosis (Toy 2009; Hosaka 2013). Studies with different NAs have demonstrated that suppression of HBV replication is associated with a significant decrease in histologic inflammatory activity and fibrosis, including partial reversion of liver cirrhosis (Mommeja-Marin 2003; Chang 2010b; Schiff 2011). With increasing treatment duration, HBeAg seroconversion rates increase, but even after 8 years of treatment they rarely exceed 40-50% of treated patients (Xing 2017). There is also evidence that effective inhibition of HBV replication can reduce HBV cccDNA, possibly parallel to the decline in serum HBsAg levels (Werle-Lapostolle 2004). As treatment of HBeAg-negative patients with NAs does not result in an endpoint in most patients even after more than a decade of therapy, new concepts are assessed. Discontinuation of long-term NA treatment may represent a novel approach to induce sustained immune control and serologic response in a significant proportion of HBeAg-negative patients (van Bömmel 2018).
As displayed in Table 5, over the last years, several NAs were approved for Hepatitis B therapy: Lamivudine (LAM), Adefovir dipivoxil (ADV), Telbivudine (LdT), Entecavir (ETV) and Tenofovir, as Tenofovir disoproxil (TDF) and Tenofovir alafenamide (TAF).
Table 5. Nucleos(t)ide analogues overview| Treatment Option | Dosage | Advantage/Disadvantage |
| Lamivudine (LAM) | 100mg/d | + cheap, generic + good availability + long-term clinical experience |
| – high rates of resistance | ||
| Adefovir (ADV) | 10mg/d | + active in LAM-resistant HBV variants |
| – weaker antiviral activity – low genetic resistance barrier – marketing license withdrawn |
||
| Telbivudine (LdT) | 600mg/d | + high antiviral activity + high rates of induced HBeAg loss |
| – cross-resistance to LAM and ADV – low genetic resistance barrier – marketing license withdrawn |
||
| Entecavir (ETV) |
0.5mg/d 1mg/d in LAM-experienced patients |
+ high resistance barrier + renal safety + cheap, generics available |
| – cross-resistance to LAM | ||
| Tenofovir disoproxil (TDF) | 245mg/d | + high resistance barrier + high antiviral activity + part of HIV antiviral regimens |
| – potential long-term side effects on renal function and bone density | ||
| Tenofovir alafenamide (TAF) | 25mg/d | + high antiviral activity + part of HIV antiviral regimens + less renal toxicity |
| – costly, no generics available to date |
Lamivudine (LAM). LAM, a (-) enantiomer of 2’ -3’ dideoxy-3’-thiacytidine, is a nucleoside analogue that was approved for the treatment of chronic HBV infection in 1988 with a daily dose of 100 mg. This dose was chosen based on a preliminary trial showing that 100 mg LAM was more effective than 25 mg and similar to 300 mg in reducing HBV DNA levels (Dienstag 1995). LAM exerts its therapeutic action when phosphorylated in the cell. By inhibiting the RNA- and DNA-dependent DNA polymerase activities, the synthesis of both the first and the second strand of HBV DNA is interrupted. Long-term LAM treatment is associated with an increasing rate of antiviral drug resistance reaching approximately 70% after 5 years in patients with HBeAg-positive HBV infections. Therefore, in many guidelines, LAM is not recommended as a first-line agent anymore. However, LAM may still play a role in combination regimens or patients with mild CHB expressing low levels of HBV DNA. An early and complete virologic response to LAM within 6 months of therapy, reaching less than 400 copies/mL is a prerequisite for long-term control of HBV infection without the risk of resistance development.
Adefovir dipivoxil (ADV). Adefovir dipivoxil was approved for the treatment of chronic Hepatitis B in the US in 2002 and in Europe in 2003. It is an oral diester prodrug of adefovir, an acyclic nucleotide adenosine analogue. It is active in its diphosphate form. ADV was the first substance with simultaneous activity against wild-type, pre-core mutated and LAM-resistant HBV variants. In vitro, it shows activity against various DNA viruses other than HBV and retroviruses (i.e. HIV). The dose of 10 mg per day was derived from a study comparing 10 mg versus 30 mg/d. The higher dosage results in stronger suppression of HBV DNA levels but is also associated with renal toxicity and an increase in creatinine levels (Marcellin 2003). ADV was the first acyclic nucleotide that was widely used in the treatment of LAM-resistant HBV infections. However, the antiviral efficacy of ADV in the licensed dosage of 10 mg/day is weaker in comparison to other available antivirals, making it more vulnerable to HBV resistance (Hadziyannis 2006b). Thus, ADV should not be used as first-line monotherapy.
Telbivudine (LdT). Telbivudine is a thymidine analogue with activity against HBV, but it is at least in vitro not active against other viruses, including HIV and Hepatitis C Virus (HCV). LdT at 600 mg/day expresses higher antiviral activity than LAM at 100 mg/day or ADV at 10 mg/day. More patients achieve HBeAg loss within 48 weeks compared to other NAs. LdT was reported to have a good safety profile at a daily dose of 600 mg/day, being non-mutagenic, non-carcinogenic, non-teratogenic, and causing no mitochondrial toxicity (Lai 2007; Hou 2008). However, elevations in creatine kinase (CK) levels were observed more often than in the group treated with LAM and neurotoxicity may be an issue when LdT is administered in combination with PEG-INF α (Fleischer 2009). Higher CK levels were also observed in the GLOBE trial comparing LdT to LAM. However, rhabdomyolysis was not seen in patients and overall treatment efficacy was higher in LdT (Liaw 2009). High rates of peripheral neuropathy were reported in patients who received combination therapy of PEG-INF α and LdT but not in patients who received LdT monotherapy (Marcellin 2015). Resistance to LdT occurs in up to 20% of patients after 2 years of treatment, predominantly in those who did not achieve undetectable HBV DNA within 6 months (Zeuzem 2009). LdT shows cross-resistance to LAM and ETV. It should not be used in LAM or ETV refractory patients. Currently, LdT is not available in most areas, since the marketing authorisation is discontinued by the FDA and the EMA at the request of the manufacturer.
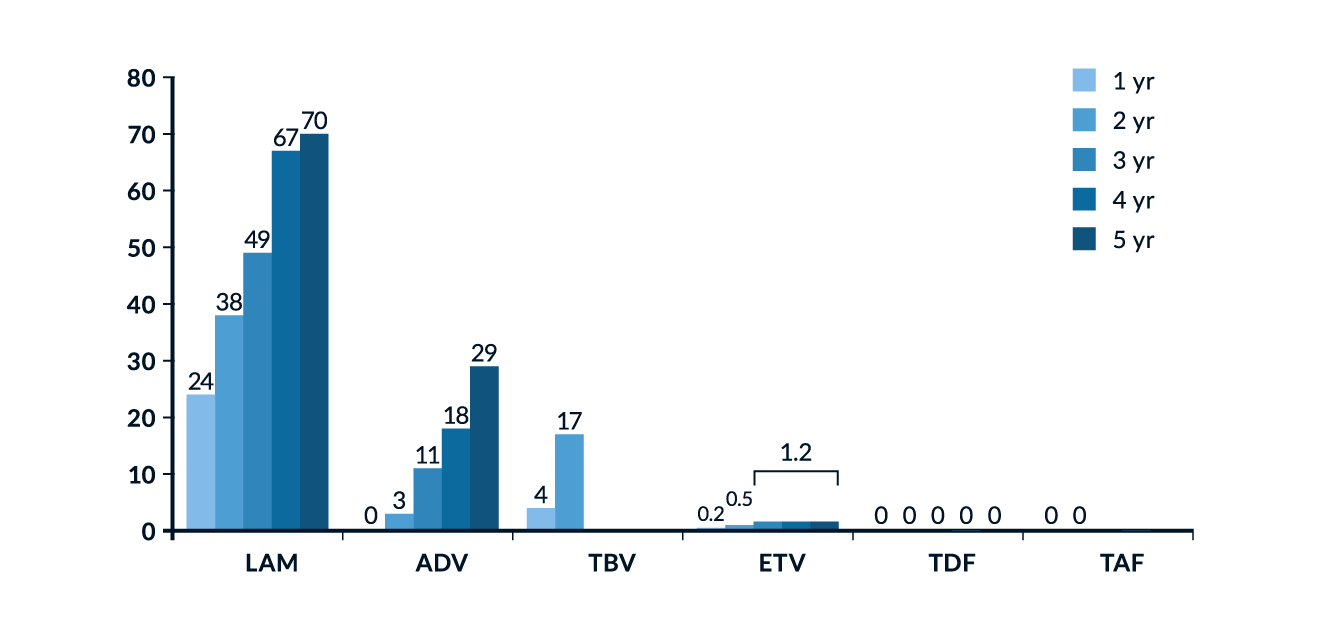 Figure 3. Cumulative incidence of HBV resistance for lamivudine (LAM), adefovir (ADV), entecavir (ETV), telbivudine (TBV), tenofovir (TDF) and tenofovir alafenamide (TAF) after several years of treatment (Collation of available data). Figure reproduced from EASL Clinical Practice Guidelines (EASL 2017).
Figure 3. Cumulative incidence of HBV resistance for lamivudine (LAM), adefovir (ADV), entecavir (ETV), telbivudine (TBV), tenofovir (TDF) and tenofovir alafenamide (TAF) after several years of treatment (Collation of available data). Figure reproduced from EASL Clinical Practice Guidelines (EASL 2017).
Entecavir (ETV). Entecavir, a cyclopentyl guanosine nucleoside analogue, is a selective inhibitor of HBV replication and was approved in 2006. Entecavir blocks all three polymerase steps involved in the replication process of the Hepatitis B virus: base priming, reverse transcription of the negative strand from the pregenomic messenger RNA and synthesis of the positive strand of HBV DNA. ETV is more efficiently phosphorylated to its active triphosphate compound by cellular kinases than other NAs. It is a potent inhibitor of wild-type HBV but is less effective against LAM-resistant HBV mutants. Therefore, ETV was approved at a dose of 0.5 mg per day for treatment-naïve HBeAg-positive and HBeAg-negative patients, but at a dose of 1 mg per day for patients with prior treatment with LAM (Chang 2005; Sherman 2008). Treatment-naïve HBeAg-positive patients achieved undetectable HBV DNA levels in 67%, 74% and 94% after one, two and five years of therapy, respectively (Chang 2010a). A virological response can be induced in over 90% of patients within one year (Lampertico 2010) and maintained in most patients over time (Hou 2020). So far, the rate of resistance at six years of treatment is estimated to be approximately 1.2% for treatment-naïve patients (Tenney 2009). Loss of HBsAg occurs in approximately 5% of treatment-naïve individuals after two years of ETV therapy (Gish 2010). In LAM-resistant patients, ETV is less potent. Fewer than 50% of patients with LAM resistance achieve undetectable HBV DNA levels after one or two years of treatment (Sherman 2008). Due to that cross-resistance, up to 45% of patients with LAM resistance develop resistance against ETV after 5 years of treatment (Tenney 2009). ETV has a favourable tolerability profile and can be easily adjusted to renal function. However, ETV may cause severe lactic acidosis in patients with impaired liver function and a MELD score of 18 points or more (Lange 2009).
Tenofovir (TFV). Tenofovir is available in two different formulas. It is an acyclic nucleoside phosphonate, or nucleotide analogue, structurally closely related to ADV. TFV has selective activity against retroviruses and hepadnaviruses and is approved for the treatment of HIV and HBV infection.
Tenofovir disoproxil fumarate (TDF), an ester prodrug form of Tenofovir (PMPA; (R)-9-(2-phosphonylmethoxypropyl) showed marked antiviral efficacy over eight years in almost all treatment-naïve HBeAg-negative and -positive patients. HBeAg loss and HBeAg seroconversion were found in 54% and 40% of patients respectively. Of the HBeAg-positive patients remaining under observation, 11.8% experienced HBsAg loss (Buti 2015). Other clinical studies show high efficacy of TDF in LAM-resistant HBV (van Bömmel 2010). Due to a possibly existing cross-resistance to ADV, the efficacy of TDF might be lowered by the presence of ADV resistance in patients with high HBV viraemia; however, a breakthrough of HBV DNA during TDF treatment in patients with previous ADV failure or in treatment-naïve patients has not been observed (van Bömmel 2010; Berg 2014). TDF is generally well tolerated and not associated with severe side effects. Renal safety during TDF monotherapy was investigated in several studies. Long-term TDF application was not associated with severe adverse outcomes concerning renal function (Heathcote 2011; Woldemedihn 2023). However, surrogate parameters of renal function changed in around 1% of patients treated with TDF, especially in patients with preexisting renal impairment (Buti 2015). In addition, effects on bone density are observed in real-world cohorts of people treated with TDF (Yip 2024).
Tenofovir alafenamide fumarate (TAF or (9-[®-2-[[(S)-[[(S)-1-(isopropoxycarbonyl) ethyl]amino] phenoxyphosphinyl]methoxy]propyl]adenin), was approved for the treatment of HBV infections in 2016. TAF follows a novel pro-drug mechanism of action and has a higher bioavailability and increased plasma stability compared to TDF. As a result, a lower daily dose of 25 mg (vs. 245 mg for TDF) is as effective as the TDF formulation in patients, regardless of the HBeAg status. The TAF formulation of Tenofovir is associated with fewer negative effects on bone and kidney biomarkers (Buti 2016; Agarwal 2018; Da Wang 2023). A switch from TDF to TAF may improve these biomarkers (Chan 2024). However, the clinical relevance of this observation remains under debate. To date, generic forms of TAF are not yet available.
Choosing the right treatment option
Interferon or NA
Initially, all patients with HBV viraemia can be considered potential candidates for interferon therapy. Because of limited tolerability and more adverse events, these patients need to be carefully selected. PEG-interferon should be preferred over standard IFN, due to its easier handling. Current guidelines recommend the use of PEG-IFN only in mild to moderate CHB (EASL 2017; Terrault 2018). Contraindications for PEG-IFN therapy include decompensated liver cirrhosis, acute Hepatitis B, autoimmune disease, uncontrolled psychiatric disease, cytopenia, severe cardiac disease or uncontrolled seizures (Terrault 2018). The potential benefit of PEG-IFN is a higher rate of HBeAg loss, HBsAg loss and long-term sustained suppression of HBV replication compared to NAs. The treatment duration of PEG-IFN is limited to 48 weeks, the benefits of the therapy often occur after treatment discontinuation. However, if a patient does not fulfil the criteria for a higher likelihood of response to treatment with PEG-INF α, has contraindications or is intolerant to PEG-INF α, long-term therapy with an NA is recommended.
Which NA?
NAs are orally administered and can achieve suppression of HBV DNA in almost all patients, but they have to be used for an undefined period unless one of the endpoints is achieved. Planned discontinuation of long-term NA treatment represents a novel approach to induce immune control in HBeAg-negative patients. The efficacy of NAs can be hampered by the emergence of HBV resistance. If an NA is chosen, several parameters have to be considered prior to therapy: the antiviral efficacy of the drug, the resistance barrier, potential side effects and the stage of liver disease. Table 5 provides an overview of the advantages and disadvantages of each NA. The preferred regimens are ETV, TDF or TAF as monotherapies. These first-line treatments are recommended in guidelines due to their strong antiviral efficacy and low rate (ETV) or to date even absence (TDF, TAF) of reported resistance (Sarin 2016; EASL 2017; Terrault 2018; WHO 2024). LAM is still licensed, but due to its weaker antiviral performance and substantial risk of resistance development, it is no longer recommended for treating CHB. The approval for LdT and ADV by EMA and FDA was withdrawn at the manufacturer’s request due to economic reasons, therefore these substances are hardly available now. Both substances should not be used in clinical routine. If a patient is already on treatment with good virological response, shows no signs of disease progression and has good adherence, the continuation of a LAM therapy can be considered, however, current guidelines give no formal recommendation for this (WHO 2024).
ETV, TDF or TAF?
Except for patients with cirrhosis, the HCC risk reduction in older and newer NAs is comparable, but ETV, TDF and TAF have a higher resistance barrier. Due to possible cross-resistance, Entecavir should be used at a higher dose of 1mg/day in LAM-experienced patients. However, if LAM resistance is confirmed, TAF or TDF should be preferred. Both formulas of Tenofovir perform equally in their antiviral activity (Lim 2023), in terms of HCC risk reduction, they are superior to ETV (Choi 2023), see Figure 4. Due to rare adverse outcomes in renal function and bone density under long-term TDF therapy, TAF or ETV should be considered in patients with present renal dysfunction or bone diseases, such as an increased risk for osteoporosis (WHO 2024). Due to the currently indefinite treatment period for many patients, therapy costs may play a role: ETV and TDF are available as generics.
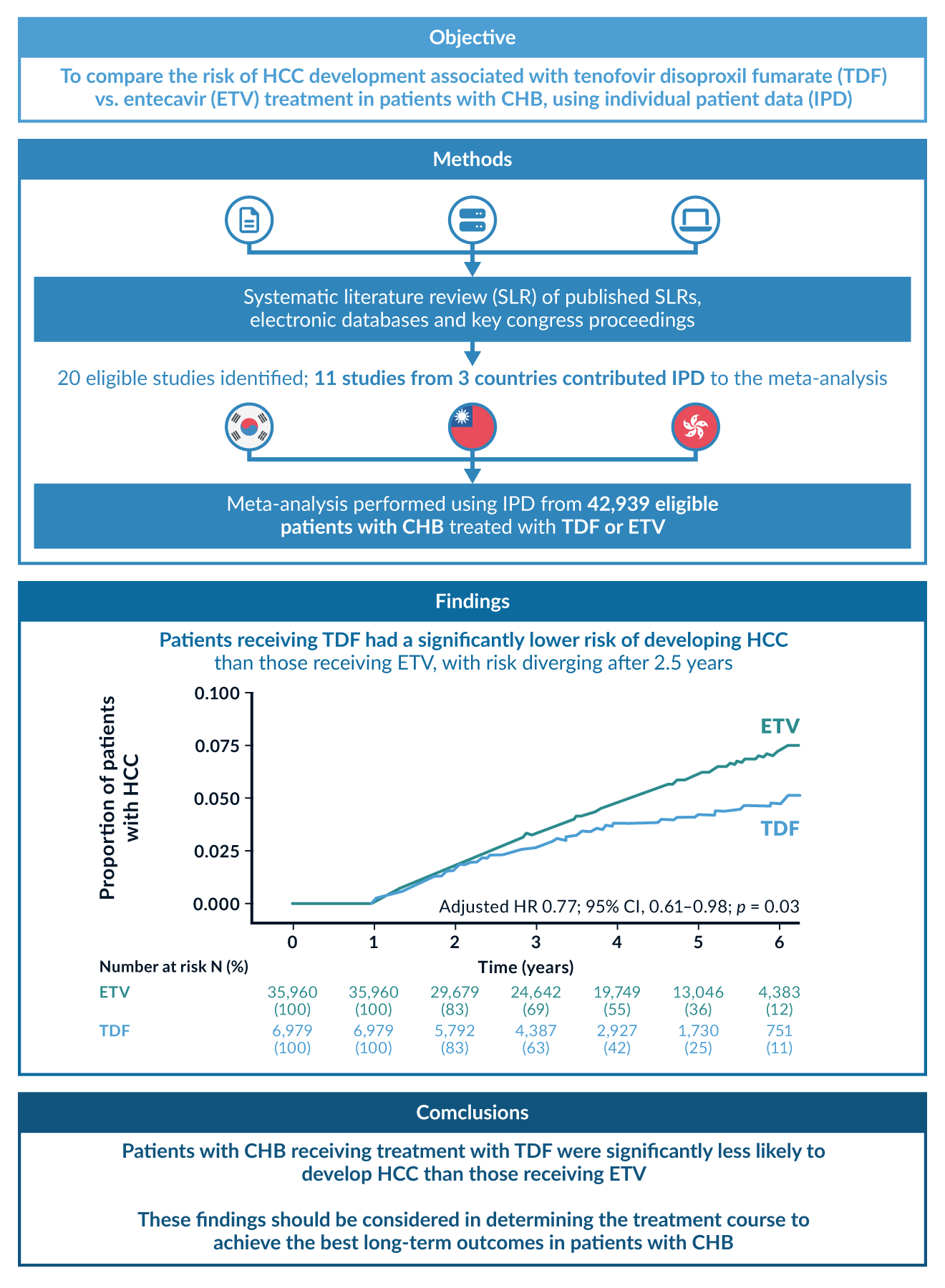 Figure 4. Comparison of HCC risk in patients treated with Tenofovir disoproxil (TDF) or Entecavir (ETV). The risk in the TDF group was significantly lower, especially for HBeAg-positive patients. Figure reproduced from a metanalysis with 40, 000 Asian patients (Choi 2023).
Figure 4. Comparison of HCC risk in patients treated with Tenofovir disoproxil (TDF) or Entecavir (ETV). The risk in the TDF group was significantly lower, especially for HBeAg-positive patients. Figure reproduced from a metanalysis with 40, 000 Asian patients (Choi 2023).
Combination therapy
Combination treatments with different NAs or NAs with PEG-IFN α were studied in various patient cohorts. However, in most trials, combinations were not superior to monotherapies, and due to insufficient knowledge of how to select patients who will benefit from first-line combination treatments, they are currently not recommended by guidelines.
NA+NA
Combining two (or more) nucleos(t)ide analogues is not superior to available monotherapies. Studies investigating combinations of LAM with ADF or LdT showed no difference in virological or biochemical response (Lai 2005; Sung 2008). In another trial, treatment-naïve patients were randomised to receive either ETV 0.5 mg/day as monotherapy or in combination with TDF. By week 96, a higher proportion of patients in the combination therapy arm showed HBV DNA suppression, the subgroup of HBeAg-positive patients with a high baseline viraemia benefited most (Lok 2012). The addition of Emtricitabine to TDF led to a higher proportion of patients with complete HBV DNA suppression in HBeAg-positive patients. However, HBeAg seroconversion or HBsAg loss was reported in only a few patients, and this was not different across both groups (Chan 2014). In ADV pre-treated patients, TDF monotherapy was as effective as the combination of TDF and Emtricitabine (Berg 2010). Although combination therapy theoretically may be useful for certain patients, especially those with incomplete response to first-line antivirals, it is currently not recommended for de-novo treatment (EASL 2017; Terrault 2018). However, the WHO guideline acknowledges that in some countries the availability of TDF plus Emtricitabine or LAM is better than TDF monotherapy due to cheaper supply as part of subsidised HIV treatment programmes. In this case, those combinations may be used for first-line therapy (WHO 2024).
NA+IFN
Although a combination of NAs and PEG-IFN α theoretically represents a more promising approach as two different mechanisms of action could potentially be synergistic, the results from clinical studies do not fully support this strategy. A stronger on-treatment virologic response at week 48 of treatment was observed with combination therapy compared to LAM or PEG-IFN α alone in one study (Chan 2005). However, a combination of LAM plus PEG-IFN α failed to demonstrate serologic or clinical benefit when evaluated at the end of follow-up in most studies (Janssen 2005). Combination therapies of PEG-IFN α with more potent NAs such as ETV or TDF may be more attractive. A combination treatment of ETV and PEG-IFN 2α after 4 years of complete response to ETV was superior to the continuation of ETV treatment by HBeAg and HBsAg loss and seroconversion rates (Ning 2014). A randomised study investigating the efficacy of either PEG-IFN α or TDF alone or in combination showed that patients treated with TDF plus PEG-IFN 2α for 48 weeks achieved significantly higher rates of HBsAg loss at week 72 (9.1%) than patients treated with either TDF (0%) or PEG-IFN 2α (2.8%) (Marcellin 2016). Despite the few promising results, evidence on combination treatment is still scarce and the risk of adverse events is higher in those therapeutic regimens, therefore current guidelines do not recommend a de-novo combination of NA and IFN (EASL 2017; Terrault 2018; Cornberg 2021).
Management of HBV resistance
Resistance development. NAs perform their antiviral action by competitive inhibition of the HBV polymerase. During treatment with these substances, HBV variants bearing mutations within the HBV polymerase gene may be selected from the HBV quasispecies, a phenomenon defined as genotypic resistance. In contrast, phenotypic resistance is defined as decreased susceptibility (in vitro testing) to inhibition by antiviral drugs associated with genotypic resistance. Cross-resistance of HBV to antiviral treatment has been described within the groups of nucleoside and nucleotide analogues. If a resistant HBV quasispecies predominates due to selective advantage, treatment might fail and a viral breakthrough during treatment may appear. This is associated with severe and sometimes fatal reactivation (Zoulim 2012). Theoretically, all available NAs may select resistant HBV strains, but resistance is rare in treatment-naive patients who receive substances with strong antiviral activity, i.e., TDF or ETV. Resistance rates against LdT, ADV and especially LAM are significantly higher. For patients treated with TDF, problems with resistance have not been reported yet, even in patients who were pretreated with ADV, although ADV resistance-associated mutations might slightly decrease response to TDF (van Bömmel 2012; Berg 2014). Global monitoring for Tenofovir resistance is necessary for the early detection of emerging TDF-resistant strains (Lumley 2024).
Detection of HBV resistance. Generally, a confirmed relapse of HBV DNA over 1 log10 from nadir during treatment with nucleoside/nucleotide analogues is considered a potential viral breakthrough caused by HBV resistance. Genotypic resistance testing is not available to most treating physicians and is generally not recommended in the first place. If available, molecular resistance testing might be considered for individuals with suspected resistance to any first-line antiviral treatment. It should be performed by a reference laboratory (Terrault 2018; Cornberg 2021; WHO 2024). It should be considered that most viral breakthroughs in treatment-naive patients receiving ETV or TDF are the result of adherence issues. Therefore, patient adherence should be assessed before genotypic resistance testing.
Avoidance of HBV resistance. HBV resistance occurs most frequently in patients treated with LAM, LdT or ADV, therefore many guidelines discourage physicians from using these NAs in first-line treatment. The selection of resistant HBV strains is more likely if HBV DNA levels are not suppressed to undetectable levels within 6 months of treatment. Therefore, in patients undergoing treatment with these substances, who show detectable HBV DNA after 6 to 12 months of treatment, the treatment should be adjusted (EASL 2017). First-line treatment with ETV or TDF/TAF is recommended by many guidelines to avoid HBV resistance (EASL 2017; Terrault 2018; WHO 2024).
Treatment of HBV resistance. Generally, resistance against a nucleoside analogue should be treated with a nucleotide analogue and vice versa. In real life, treatment with TDF has shown effectiveness against most kinds of HBV variants associated with resistance against either nucleoside or nucleotide analogues. Thus, a switch to monotherapy with TDF was shown to be very effective in patients with resistance to LAM and also in patients with resistance to ADV in European and Asian patients (van Bömmel 2010; Huang 2017). In a randomised study, patients with resistance to LAM did not show a better response to a combination treatment of TDF plus Emtricitabine compared to TDF monotherapy (Fung 2014). In another study, it was observed that monotherapy with TDF was superior to Entecavir-Adefovir combination treatment in NA-resistant patients with suboptimal response to Lamivudine-Adefovir (Lee 2018). Thus, most guidelines recommend a switch to TDF or TAF in patients with treatment failure due to resistance (EASL 2017; WHO 2024), see Table 6. The combination of TDF with a nucleoside analogue might be useful in patients with multiple pre-treatments who have accumulated different resistance mutations (Petersen 2012; van Bömmel 2012). If a Tenofovir resistance is suspected, the addition of ETV may be considered, however, due to the rarity of this event in real-world settings, evidence about the efficacy is scarce.
Table 6. Management of patients with NA resistance. Recommendations on alternative regimes (switching). Table reproduced from EASL Clinical Practices Guidelines (EASL 2017).| Resistance pattern | Recommended rescue strategies |
| LAM resistance | Switch to TDF or TAF |
| TBV resistance | Switch to TDF or TAF |
| ETV resistance | Switch to TDF or TAF |
| ADF resistance | If LAM-naïve: switch to ETV or TDF or TAF If LAM-resistance: switch to TDF or TAF If HBV DNA plateaus: add ETV*** or switch to ETV |
| TDF or TAF resistance** | If LAM-naïve: switch to ETV If LAM-R: add ETV* |
| Multidrug resistance | Switch to ETV plus TDF or TAF combination |
LAM = lamivudine; ADV = adefovir, TBV = telbivudine.
* The long-term safety of these combinations is unknown.
** Not seen clinically so far; do genotyping and phenotyping in an expert laboratory to determine the cross-resistance profile.
*** Especially in patients with ADV resistant mutations (rA181T/V and/or rN236T) and high viral load, the response to TDF (TAF) can be protracted.
Treatment Monitoring (How to monitor treatment?)
Baseline
Prior to the initiation of therapy, baseline parameters should be measured. The number of recommended tests varies among different guidelines and needs to be adjusted according to local circumstances (EASL 2017; Cornberg 2021; KASL 2022; WHO 2024).
Virological tests
- Quantitative HBV DNA levels, measured with a highly sensitive assay
- HBsAg, ideally with a quantitative assay
- HBeAg
- Anti-HBe
- Anti-HBs and anti-HBc may play a role in the initial diagnosis of HBV infection
- Screening for concomitant viral infections (HIV, HCV, HDV)
HBV genotyping is only recommended in patients who are considered candidates for treatment with IFN. HBV resistance testing can be useful in patients with prior failure to more than one NA, but this is not a standard diagnostic approach.
General lab tests
- Serum levels of alanine transaminase (ALT) and other liver function tests
- Kidney function tests
- complete blood count
- Assessment of liver parenchyma status
- Ultrasound imaging
- Non-invasive fibrosis assessment: transient elastography, APRI-Score
- Liver biopsy and histology: no routine use
Under therapy
During therapy, HBV DNA, ALT and creatinine levels should be measured after 4 to 6 weeks and later every 3 months. The early identification of viral resistance is crucial to adjust the therapy if necessary. Patients with a stable suppression of HBV replication to levels below 300 copies/mL (60 IU/mL) and no signs of severe liver damage may be scheduled at 6-month surveillance intervals. HBsAg and, in HbeAg-positive patients, HBeAg and anti-HBe should also be measured once HBV DNA levels have become undetectable, to detect serologic response and therapeutic endpoints. When using TDF as a therapeutic regime, renal function tests and regular assessment of bone density might be helpful to detect long-term side effects of treatment.
HCC risk
The risk for HCC development remains increased even in patients with complete viral suppression during long-term treatment with NA. However, identifying those patients with a greater risk and the necessity for more regular monitoring remains challenging. Scoring systems can help estimate the individual risk of HCC development. Several scoring systems have been proposed to monitor the HCC risk during NA treatment including the HCC-Rescue, CAMD and mREACH-B score. Most risk scores were developed and tested using Asian cohorts, they perform almost equally. However, a recent meta-analysis favoured the HCC-Rescue score in terms of clinical practicability and risk group discrimination (Xu 2023). For European individuals, the PAGE-B score, which is based on different parameters, seems to allow a more precise prediction as compared to the other scores (Papatheodoridis 2016a). The newly developed aMAP score underwent a validation process with patient groups of different ethnicities and with different forms of hepatitis. Even non-viral hepatitis was included. It showed a good discriminatory ability and calibration and could therefore be useful in various clinical settings worldwide (Fan 2020). A comparison of selected risk scores can be found in Table 7. The scores with their corresponding cut-offs may help to determine, which CHB patients have an elevated risk for HCC development. These patients, along with other high-risk subgroups (cirrhosis, family history of HCC) should be subject to regular screening, including ultrasound imaging and measuring of AFP levels (WHO 2024).
Table 7. HCC risk scores (under treatment)| Score | Parameters | Cohorts | Cut-Off* | Publication |
| HCC-Rescue | age, sex, presence of cirrhosis | Asian patients | 65/85 | (Sohn 2017) |
| APA-B | age, AFP, platelet count | Asian patients | 6/10 | (Chen 2017) |
| mREACH-B | age, sex, ALT, liver stiffness, HBeAg status | Asian patients | – | (Lee 2014) |
| PAGE B | age, sex platelet count | European patients | 10/18 | (Papatheodoridis 2016a) |
| CAMD | age, sex, presence of diabetes mellitus, presence of cirrhosis | Asian patients | 8/14 | (Hsu 2018) |
| aMAP | age, sex, albumin, total bilirubin, platelet count | Asian and European patients, also treated HCV patients | 50/60 | (Fan 2020) |
Prognostic factors and treatment response
Effective treatment of HBV ideally reaches defined endpoints and results in a reduction of overall disease burden. It is important to assess the treatment response, regardless of the form of treatment used.
Criteria for treatment response:
Virologic response
- Sustained decrease of HBV DNA, to at least <2, 000 IU/mL (corresponding to <10, 000 copies/mL), ideally to <60 IU/mL (<300 copies/mL)
- Sustained HBeAg seroconversion in former HBeAg-positive patients
- Ideally: loss of HBsAg with or without the appearance of anti-HBs
Biochemical response
- Sustained ALT normalisation
Histologic response
- Reduction of fibrosis (histological staging)
- Reduction of inflammatory activity (histological grading).
Potential long-term effects
- Avoidance of cirrhosis, hepatocellular carcinoma (HCC), transplantation and death
Baseline factors: Several factors are associated with long-term remission and may help to guide treatment decisions. Pre-treatment factors predictive of HBeAg seroconversion are low viral load, high ALT levels (above 2-5 x ULN) and high histological grading (Wong 1993; Perrillo 2002; Flink 2006; Lai 2007; Yuen 2007; Buster 2009). These general baseline predictors are particularly relevant for treatment regimens with PEG-IFN α but may be in part also for NAs. A pooled analysis from the two largest trials using PEG-IFN α-2a or -2b in CHB tried to calculate a score predicting successful interferon therapy based on an individual patient’s characteristics (viral load, ALT level, HBV genotype, age, gender). However, this approach may only be feasible in HBeAg-positive patients. (Buster 2009).
HBV genotypes: HBV genotypes are associated with IFN α treatment success. Patients with HBV genotype A, prevalent in northern Europe and the US, show a much higher rate of HBeAg and HBsAg seroconversion than patients with HBV genotype D, prevalent in the south of Europe, or the HBV genotypes B or C originating from Asia (Flink 2006; Keeffe 2007). During treatment with nucleos(t)ide analogues, suppression of HBV replication and induction of HBeAg loss can be achieved regardless of the present genotype. However, HBsAg loss was almost exclusively observed in patients with genotypes A or D.
HBV DNA: During antiviral therapy, the decrease of HBV DNA levels from baseline is the most important tool in monitoring treatment efficacy. A complete response to antiviral therapy is defined as the suppression of HBV DNA below the limit of detection as measured by a sensitive real-time PCR assay. Incomplete suppression is characterised by persistent HBV replication despite antiviral therapy. Ongoing HBV replication in the presence of the drug should be avoided to prevent the selection of resistant HBV strains in the so-called “plateau phases”. A breakthrough of HBV DNA during continuous NA treatment may be caused by viral resistance; however, if NAs with high genetic barriers against resistance such as ETV or TFD are used, non-adherence to the antiviral treatment is more likely. Measuring HBV DNA kinetics early during therapy will help guide antiviral treatment and establish early stopping rules or add-on strategies to avoid antiviral failure.
An incomplete or partial virologic response to NAs is defined as a decrease of HBV DNA of more than 1 log10 IU/mL but remaining measurable (Lavanchy 2004). The timespan to reach HBV DNA suppression depends on the type of treatment: for agents with a high genetic barrier against resistance (ETV or TDF), a partial response is defined after 12 months and for substances with a low genetic barrier like LAM or LdT, after 6 months of monotherapy. In case of partial response to a drug with a low genetic barrier, an appropriate rescue therapy should be initiated. It was recently shown that patients with partial response to LAM or ADV have a high probability of responding to TDF monotherapy, without risking the development of resistance (van Bömmel 2010; Heathcote 2011; Berg 2014). For patients with partial response to a drug with a high genetic barrier such as ETV or TDF, current guidelines recommend considering the initiation of a combination treatment. However, this might be necessary only in a minority of patients, as published long-term studies have shown that the continuation of a first-line monotherapy with ETV or TDF increases the percentage of patients with undetectable HBV DNA over time without leading to resistance development (Chang 2010b; Buti 2015). Therefore, in case of incomplete viral suppression at week 48, a continuation of monotherapy with TDF or ETV 1 mg is advisable as long as HBV DNA levels decrease continuously. However, the debate on whether to switch treatment or add a second drug for optimal management is not yet resolved.
Timepoint of HBeAg-loss. In patients who were treated with PEG-IFN α-2b as monotherapy or in combination with LAM, the loss of HBeAg within the first 32 weeks of treatment was shown to be an on-treatment predictor for HBsAg loss during a mean period of 3.5 years after the end of treatment. HBsAg loss was found in 36% of the patients with early HBeAg loss and only in 4% of the patients with HBeAg loss after 32 weeks of treatment (Buster 2009).
HBsAg levels: The response of HBeAg-positive and HBeAg-negative patients to PEG-IFN treatment can be predicted by measuring HBsAg levels before and changes in HBsAg levels during treatment. During PEG-IFN treatment for HBeAg-positive chronic HBV infection, an absence of a decline in HBsAg levels at week 12 of treatment reduced the probability of response to less than 5% in one study (Sonneveld 2010). In the NEPTUNE trial investigating the predictive value of HBsAg levels in HBeAg-positive patients receiving PEG-IFN α -2a over 48 weeks, it was shown that in patients achieving suppression of HBsAg to levels below 1, 500 IU/mL after 12 weeks of treatment, the chance of reaching HBeAg seroconversion, suppression of HBV DNA to undetectable levels and HBsAg loss 6 months after treatment was higher. In patients still showing HBsAg levels over 20, 000 IU/mL after 12 weeks of treatment, none of the endpoints was achieved (Liaw 2011). Also, in HBeAg-negative patients, the decrease of HBsAg after 12 weeks of PEG-IFN α treatment can predict long-term response. This prediction can be made even more precise regarding the kinetics of both HBsAg and HBV DNA (Moucari 2009).
Treatment cessation (When to stop?)
Treatment duration and stopping rules
Treatment with modern and potent NAs usually results in a quick and durable suppression of HBV DNA replication. While there is widespread agreement among the guidelines on who to treat, it is yet under debate how long the therapy should last. The duration of NA therapy was primarily set to an indefinite length, due to the observed relapse in disease activity after short-term NA application. However, treatment discontinuation may be a novel approach to induce functional cure in a subset of patients. The recommendations about when to discontinue treatment depend on the treatment endpoint the patients have reached.
Patients with HBsAg loss
Treatment with NAs can safely be withdrawn in patients who reach the endpoint of functional cure, i.e. HBsAg loss or seroconversion to anti-HBs. This status is durable and clinical or virological reversion is rare in these patients and usually without complications (Kim 2014). The HCC risk in patients who achieve HBsAg loss under therapy seems to be much lower than those only achieving virological suppression (Yip 2019).
HBeAg seroconversion
Treatment-induced HBeAg loss or seroconversion in previously HBeAg-positive patients is one of the treatment endpoints. The seroconversion is seen as a surrogate marker for silencing HBV transcriptional activity. Current guidelines recommend a consolidation phase of at least another 12 months before stopping NAs in these patients to reduce the risk of sero-reversion (EASL 2017; Terrault 2018).
HBeAg-negative patients with detectable HBsAg
As previously described, induced HBsAg loss occurs in only around 1% of HBeAg-negative patients on treatment with NAs. Unable to reach a defined endpoint, these patients may therefore undergo an almost lifelong treatment. Although the safety of modern NAs has been proven, long-term side effects and treatment costs may be of concern in some settings. An off-treatment “cure” is desired by both patients and clinicians. While practical details are still under debate, newer guidelines acknowledge this novel approach as a possible strategy for eligible patients. Treatment discontinuation leads to a relapse in HBV replication in almost all patients, often in combination with signs of disease activity, such as increased ALT levels (Ghany 2020). Those relapses are in fact associated with a reactivation of the previously “hibernating” immune system. Some patients lose their HBsAg in the course of this immune reactivation (Tout 2021). The potential of this approach was demonstrated in the FINITE trial, where 43% of non-cirrhotic patients did not require re-therapy after TDF discontinuation, either by achieving HBsAg loss or remaining in a status with low viraemia (Berg 2017). In a randomised controlled trial (STOP-NUC), comparing NA discontinuation to ongoing treatment, 10% of the patients lost their HBsAg and 40% remained in virological remission in the discontinuation arm (van Bömmel 2023). A typical disease course after NA cessation is shoen in Figure 5.
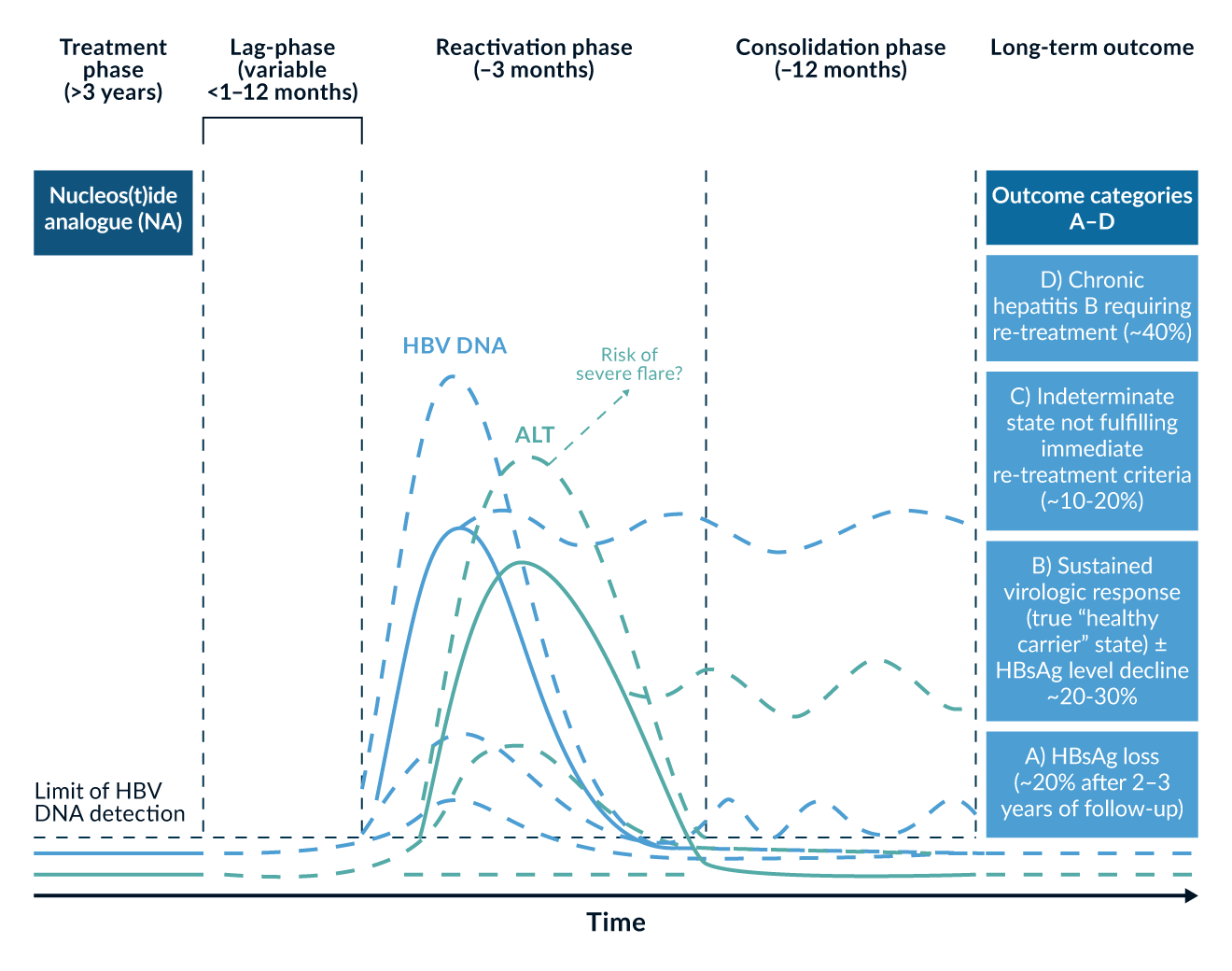 Figure 5. Dynamics of HBV DNA and ALT levels after NA treatment cessation in HBeAg-negative patients, following a period of treatment for at least 3 years. Different long-term outcomes are listed. Figure reproduced from the report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference (Cornberg 2020).
Figure 5. Dynamics of HBV DNA and ALT levels after NA treatment cessation in HBeAg-negative patients, following a period of treatment for at least 3 years. Different long-term outcomes are listed. Figure reproduced from the report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference (Cornberg 2020).
Who is eligible for a stopping NA?
There is widespread agreement that patients for this approach must be carefully selected and closely monitored, preferably in trials. If monitoring and induction of re-treatment or emergency handling of patients with a severe relapse are not guaranteed, this strategy may not be safe for the patients. The advantages and disadvantages of therapy cessation need to be carefully weighed: on the one hand, there is a higher chance of inducing HBsAg loss and functional cure, in around 10% of the patients. Even more patients proceed to a state with low disease activity, without the need for re-treatment. On the other hand, most patients experience an increase in HBV DNA and ALT levels and excellent patient adherence is required since regular clinical follow-up visits should be performed. In about half of the patients, subsequent re-treatment is necessary (Berg 2021). It is yet difficult to predict the course of the disease and the probability of reaching HBsAg loss in patients with NA discontinuation. As evidence is still scarce, universal stopping rules are not yet defined. The selection of patients that most likely benefit from this approach is currently under investigation in studies, but the first results may help to select those that most likely lose HBsAg or remain virologically suppressed. A low pre-treatment viraemia, a decrease in quantitative HBsAg under therapy and low HBsAg levels upon stopping are positive predictive markers for success (Liu 2019). Newer biomarkers such as HBV RNA and HB core-related antigen (HBcrAg) may help to further stratify the groups on treatment concerning their risk of severe relapse or more beneficial outcomes after cessation (Berg 2021). Due to the risk of severe decompensation, all patients with cirrhosis should remain on infinite NA treatment as long as HBsAg is measurable. In Figure 6, an algorithm for consideration of NA discontinuation is displayed.
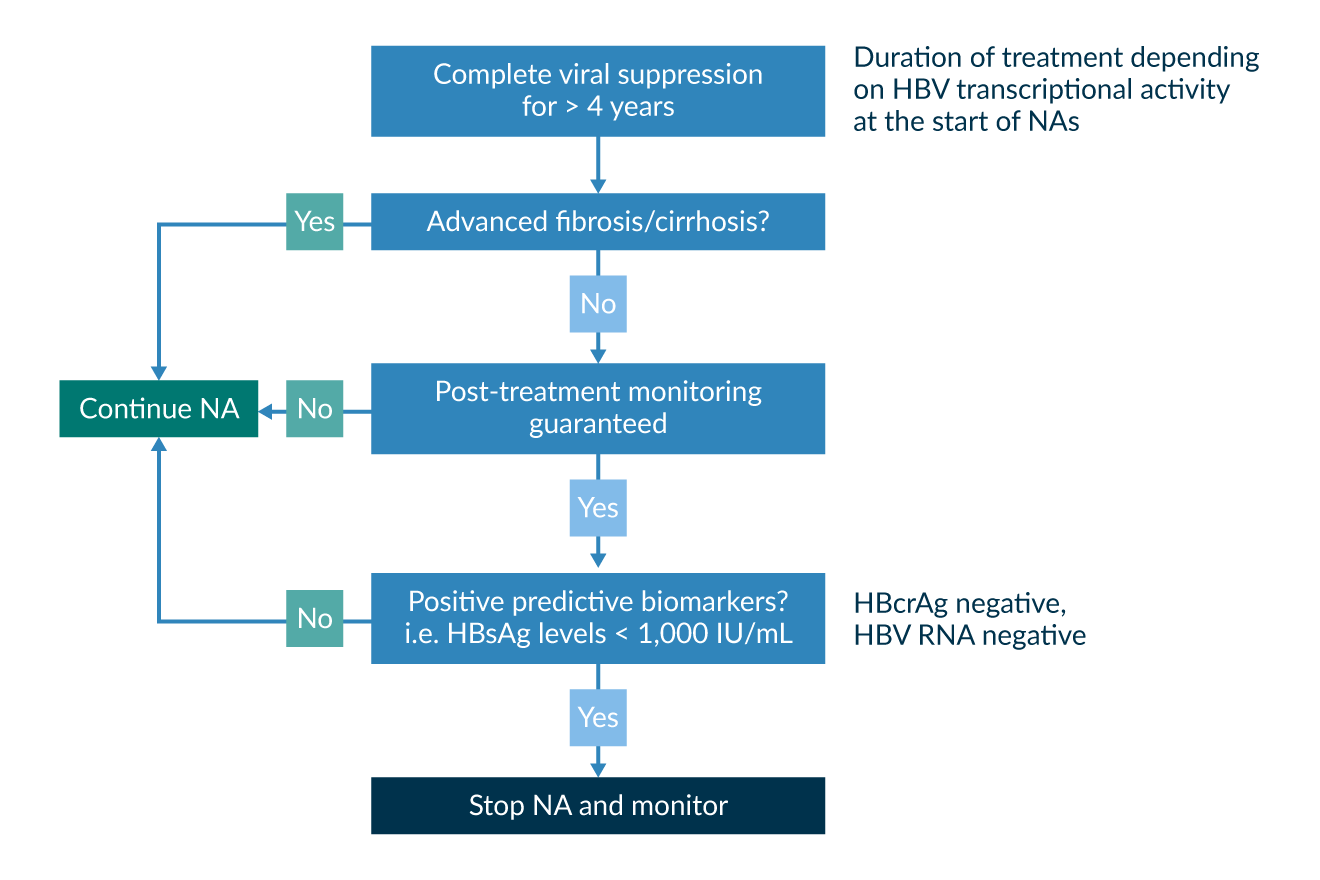 Figure 6. Proposed algorithm and decision aid for an NA treatment discontinuation approach.
Figure 6. Proposed algorithm and decision aid for an NA treatment discontinuation approach.
PEG-IFN
PEG-IFN α should be administered for 48 weeks in HBeAg-positive and HBeAg-negative patients. If no decrease in HBV DNA or/and in HBsAg levels can be noted after 12 weeks of treatment, further response is unlikely, and treatment may be stopped early in agreement with the patient.
Outlook
In today’s world, there are many strategies for preventing Hepatitis B, monitoring patients on and off treatment and finding an appropriate therapy for the vast majority of the infected. However, the complete eradication of HBV from infected individuals cannot be achieved by any of the currently available treatments, this is due to the persistence of HBV cccDNA in the hepatocytes. In the dynamic field of HBV research, many teams are working on the development of a complete, long-lasting and even “sterile” cure. In addition, new biomarkers emerge, which help to predict outcomes for recently developed treatment strategies.
New parameters for treatment monitoring
Currently, most clinicians around the world use quantitative HBV DNA, HBsAg and anti-HBs, as well as qualitative anti-HBc, HBeAg and anti-HBe for Hepatitis B diagnostics and treatment monitoring. The innovation of newer biomarkers can help to gain a deeper understanding of disease dynamics both on and off treatment, see Figure 7. Levels of Hepatitis B core-related antigens (HBcrAg) may help to predict HBsAg seroclerance (Tseng 2023). HBV RNA may act as a parameter to determine cccDNA transcriptional activity (Wang 2016), this could help to predict the outcome in patients where treatment cessation is planned (Seto 2021). The emergence of those and other biomarkers supports clinicians in further individualising and planning therapeutic approaches.
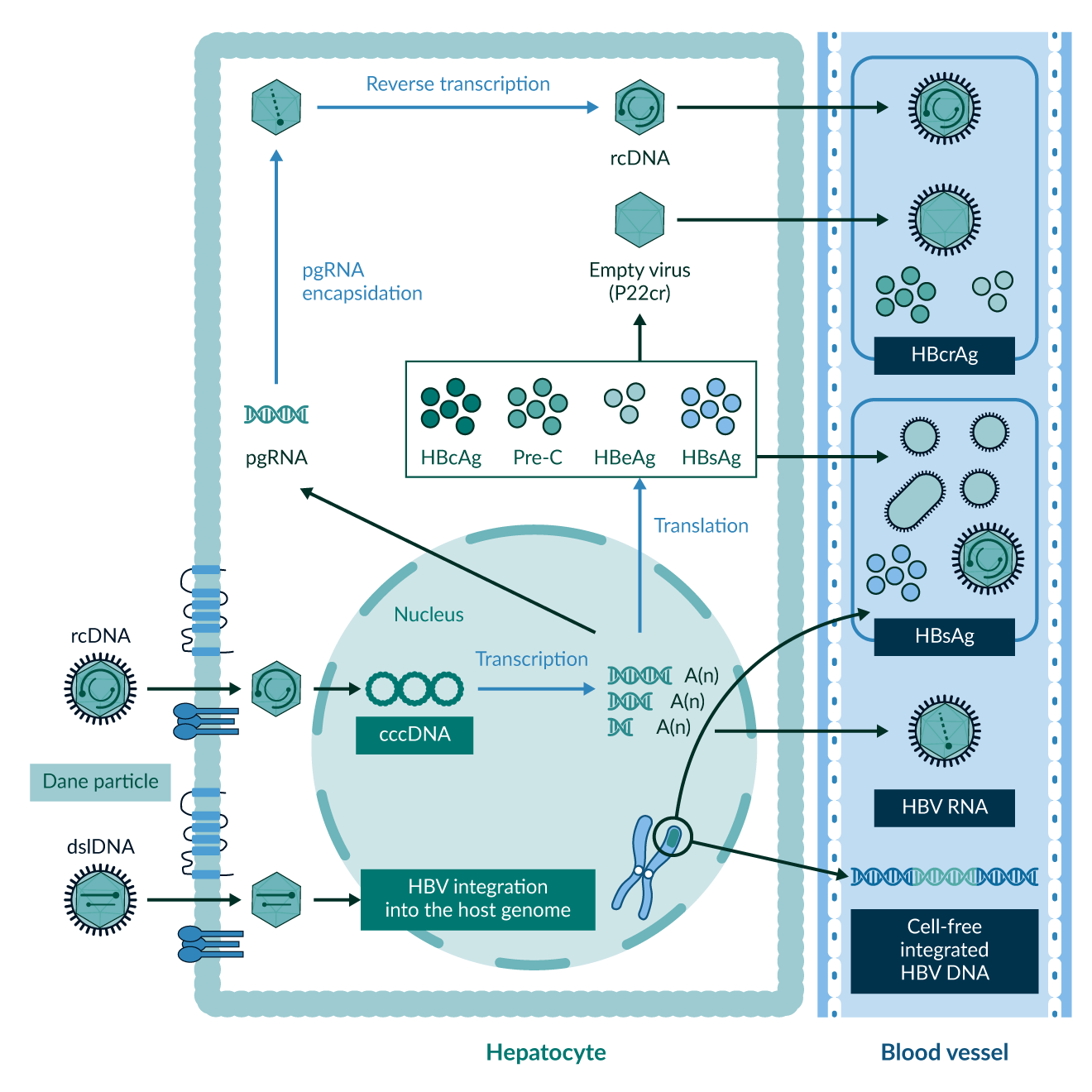 Figure 7. Overview of old and new biomarkers for disease and treatment monitoring in Hepatitis B, both in hepatocytes and bloodstream. Figure taken from KASL clinical practice guidelines for management of chronic hepatitis B (KASL 2022).
Figure 7. Overview of old and new biomarkers for disease and treatment monitoring in Hepatitis B, both in hepatocytes and bloodstream. Figure taken from KASL clinical practice guidelines for management of chronic hepatitis B (KASL 2022).
New therapeutic strategies
Even after HBsAg loss, the remainder of HBV stays in the body in the form of cccDNA. Although generally rare, HBV reactivation may occur in patients undergoing immunosuppression. In addition, some risk of HCC development remains after seroclearance (Yang 2022). A “sterile” cure with complete elimination of cccDNA is not yet achievable with current therapeutic regimes but remains the ultimate goal in new drug development. In addition, the optimisation of available antiviral options as well as new therapeutic targets to achieve HBsAg loss are similarly important. Different approaches are already being investigated and some potential candidates have reached phase II and III trials (see Table 8, Figure 8).
Table 8. New therapeutic approaches| Drug type | Mode of action |
| Capsid assembly modulators | Inhibition of capsid assembly, reduction of cccDNA expression |
| Anti-sense oligonucleotides | Inhibition of HBsAg production |
| siRNA | Silencing of viral RNA, inference with viral protein production |
| HBsAg release inhibitors | Inhibition of HBsAg assembly and release |
| Gene editing | Specific cutting and destruction of HBV DNA |
| Therapeutic vaccine | Enhancement of host immune system by exposition to antigens |
| Toll-like receptor agonists | Activation of innate immune system by inducing specific pathways |
| Checkpoint inhibitors | Reversion of T cell dysfunction |
| Monoclonal antibodies | Targeted against viral structures |
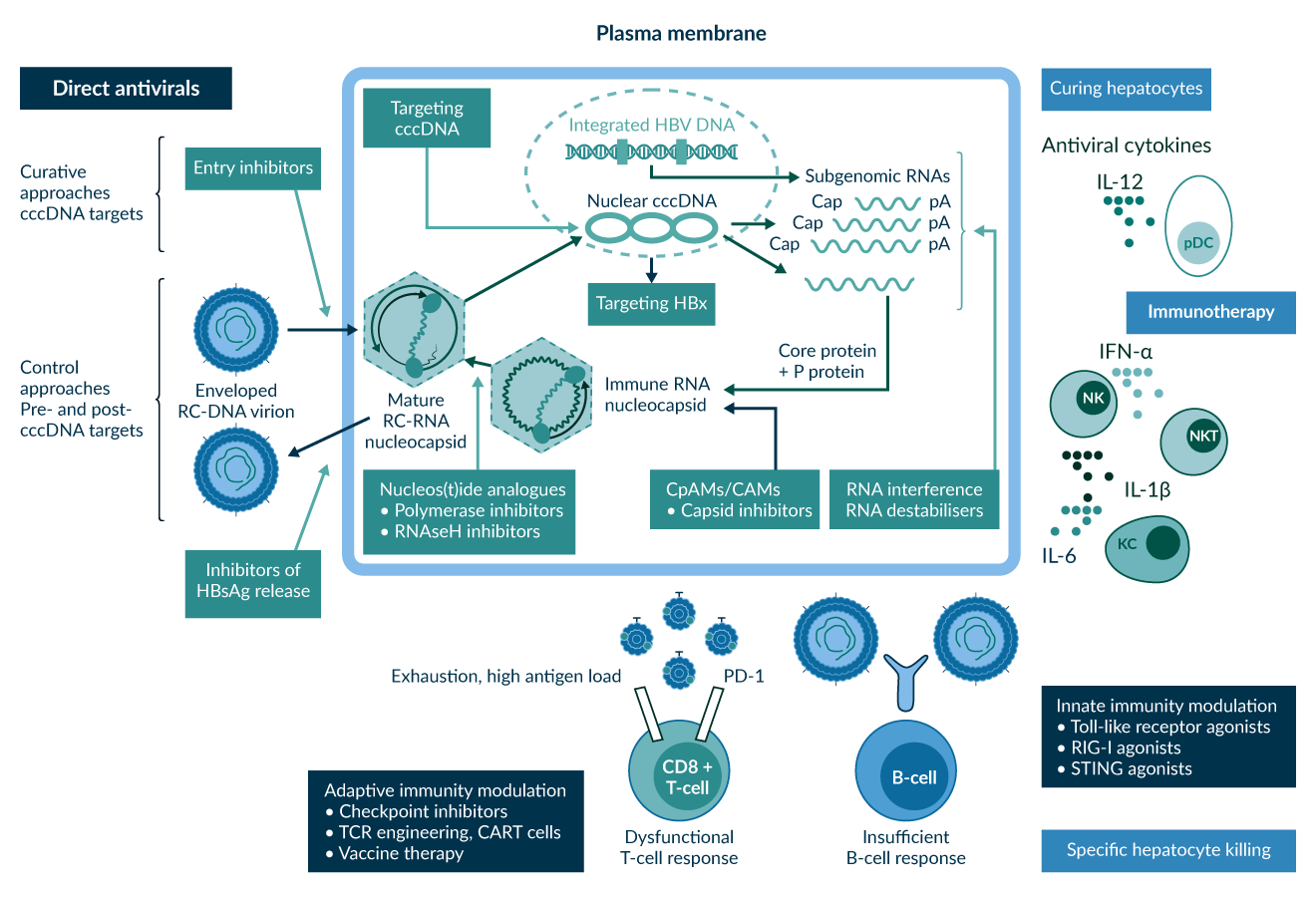 Figure 8. Overview of current and possible future therapeutic targets and corresponding drug classes. Reproduced from a strategy paper on Hepatitis B cure (Revill 2019).
Figure 8. Overview of current and possible future therapeutic targets and corresponding drug classes. Reproduced from a strategy paper on Hepatitis B cure (Revill 2019).
The combination of two or more therapeutic options directed at different targets in the viral lifecycle seems promising in terms of stronger viral suppression and immune stimulation. Complementary strategies may help to reach a functional or even sterilising cure by reducing HBV replication and HBsAg load while simultaneously reinforcing the host immune system to fulfil its role in HBV elimination (Figure 9). While NAs currently remain the backbone of therapy, first trial results of new drugs are promising and may lead to a new era of Hepatitis B treatment. Replication inhibitors, antigen reducers and immune modulators are the main three classes of new therapeutics (Figure 10). The combination of those different mechanisms of action potentially leads to a stronger antiviral activity. However, its superiority to monotherapies and long-term safety must be assessed in future trials (Feld 2023).
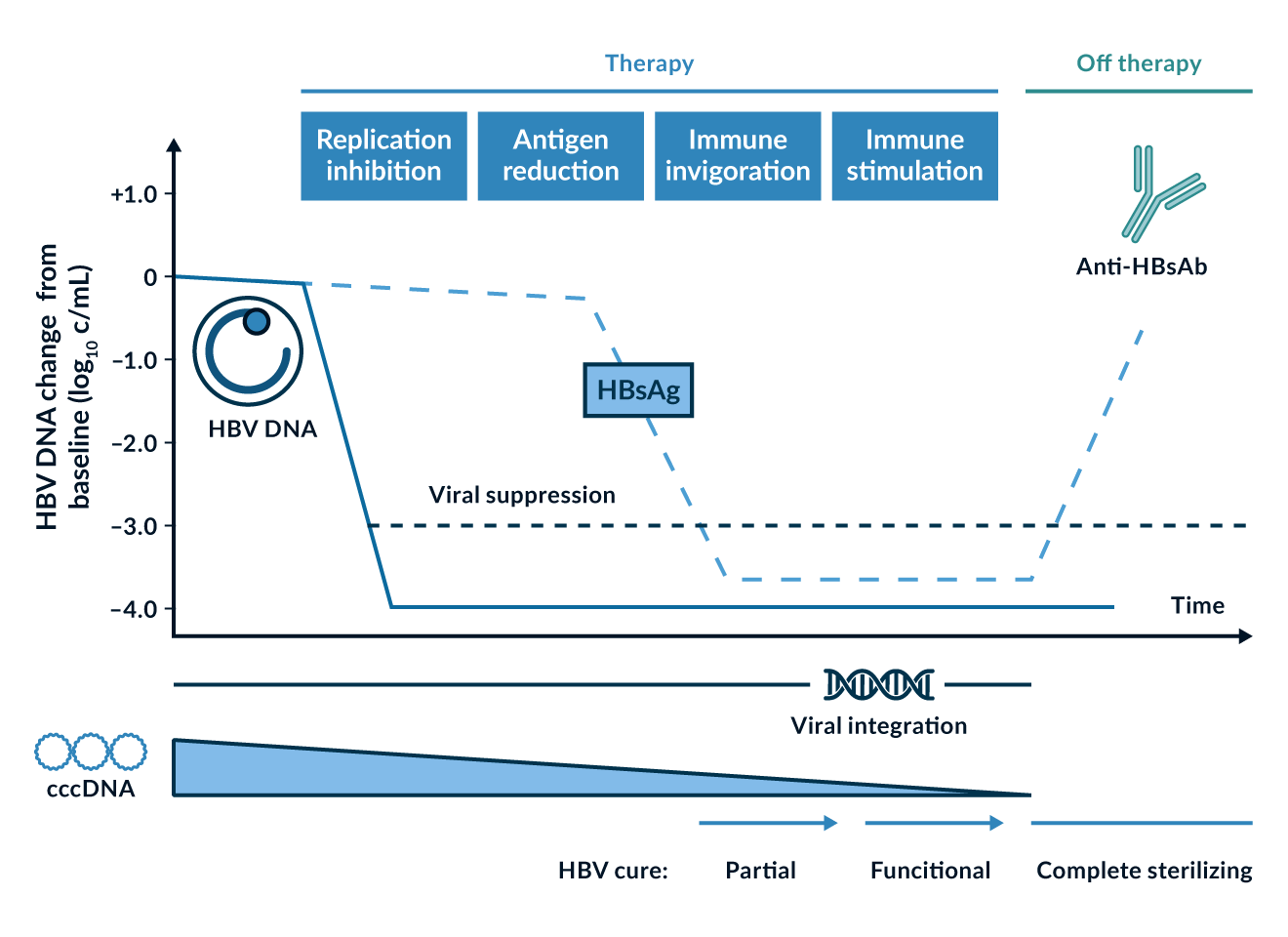 Figure 9. The potential of combination therapy with agents directed at different parts of the viral life cycle and host immune system. Figure reproduced from a review article concerning New Perspectives on Development of Curative Strategies for Chronic Hepatitis B (Feld 2023).
Figure 9. The potential of combination therapy with agents directed at different parts of the viral life cycle and host immune system. Figure reproduced from a review article concerning New Perspectives on Development of Curative Strategies for Chronic Hepatitis B (Feld 2023).
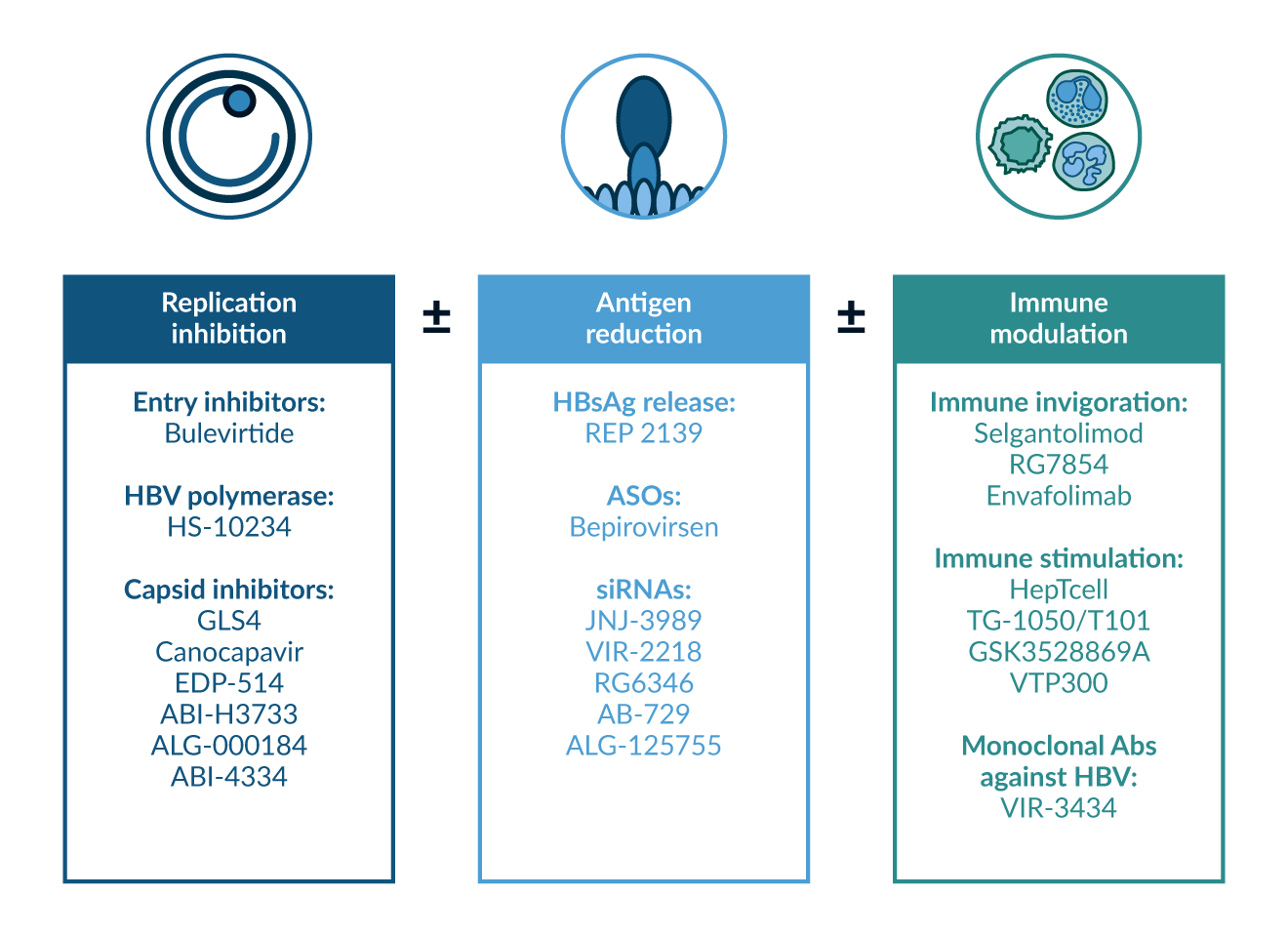 Figure 10. Different classes of new therapeutic agents and an (incomplete) list of substances currently under investigation. Figure reproduced from a review article concerning New Perspectives on Development of Curative Strategies for Chronic Hepatitis B (Feld 2023).
Figure 10. Different classes of new therapeutic agents and an (incomplete) list of substances currently under investigation. Figure reproduced from a review article concerning New Perspectives on Development of Curative Strategies for Chronic Hepatitis B (Feld 2023).
The future importance and clinical value of new therapeutic options is not yet easy to determine. The field of HBV therapy is dynamic and the list of the most promising drug candidates changes fast. Different websites have regularly updated databases on current drug development. Interested readers should pay continuous attention. The Hepatitis B Foundation displays an overview of current trials and candidates: https://www.hepb.org/treatment-and-management/drug-watch/
Elimination of Hepatitis B as a global threat and reason for disease burden remains the ultimate goal that may be achieved by concerted action in the next decades.
References
Agarwal, K., Brunetto, M. & Seto, W. K. et al. (2018) 96 weeks treatment of tenofovir alafenamide vs. tenofovir disoproxil fumarate for hepatitis B virus infection. Journal of hepatology 68 (4), 672–681.
Artz, A. S., Somerfield, M. R. & Feld, J. J. et al. (2010) American Society of Clinical Oncology provisional clinical opinion: chronic hepatitis B virus infection screening in patients receiving cytotoxic chemotherapy for treatment of malignant diseases. Journal of clinical oncology official journal of the American Society of Clinical Oncology 28 (19), 3199–3202.
Berg, T. & Lampertico, P. (2021) The times they are a-changing - A refined proposal for finite HBV nucleos(t)ide analogue therapy. Journal of hepatology 75 (2), 474–480.
Berg, T., Marcellin, P. & Zoulim, F. et al. (2010) Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology 139 (4), 1207–1217.
Berg, T., Simon, K.-G. & Mauss, S. et al. (2017) Long-term response after stopping tenofovir disoproxil fumarate in non-cirrhotic HBeAg-negative patients - FINITE study. Journal of hepatology 67 (5), 918–924.
Berg, T., Zoulim, F. & Moeller, B. et al. (2014) Long-term efficacy and safety of emtricitabine plus tenofovir DF vs. tenofovir DF monotherapy in adefovir-experienced chronic hepatitis B patients. Journal of hepatology 60 (4), 715–722.
Borg, M. J. ter, Leemans, W. F., Man, R. A. de & Janssen, H. L. A. (2008) Exacerbation of chronic hepatitis B infection after delivery. Journal of viral hepatitis 15 (1), 37–41.
Brunetto, M. R., Oliveri, F., Colombatto, P., Coco, B., Ciccorossi, P. & Bonino, F. (2003) Treatment of HBeAg-negative chronic hepatitis B with interferon or pegylated interferon. Journal of hepatology 39 Suppl 1, S164-7.
Buster, E. H. C. J., Flink, H. J. & Simsek, H. et al. (2009) Early HBeAg loss during peginterferon alpha-2b therapy predicts HBsAg loss: results of a long-term follow-up study in chronic hepatitis B patients. The American journal of gastroenterology 104 (10), 2449–2457.
Buti, M., Gane, E. & Seto, W. K. et al. (2016) Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. The lancet. Gastroenterology & hepatology 1 (3), 196–206.
Buti, M., Tsai, N. & Petersen, J. et al. (2015) Seven-year efficacy and safety of treatment with tenofovir disoproxil fumarate for chronic hepatitis B virus infection. Digestive diseases and sciences 60 (5), 1457–1464.
Chan, H. L. Y., Buti, M. & Lim, Y.-S. et al. (2024) Long-Term Treatment With Tenofovir Alafenamide for Chronic Hepatitis B Results in High Rates of Viral Suppression and Favorable Renal and Bone Safety. The American journal of gastroenterology 119 (3), 486–496.
Chan, H. L. Y., Chan, C. K. & Hui, A. J. et al. (2014) Effects of tenofovir disoproxil fumarate in hepatitis B e antigen-positive patients with normal levels of alanine aminotransferase and high levels of hepatitis B virus DNA. Gastroenterology 146 (5), 1240–1248.
Chan, H. L.-Y., Leung, N. W.-Y. & Hui, A. Y. et al. (2005) A randomized, controlled trial of combination therapy for chronic hepatitis B: comparing pegylated interferon-alpha2b and lamivudine with lamivudine alone. Annals of internal medicine 142 (4), 240–250.
Chang, T.-T., Gish, R. G. & Hadziyannis, S. J. et al. (2005) A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology 129 (4), 1198–1209.
Chang, T.-T., Lai, C.-L. & Kew Yoon, S. et al. (2010a) Entecavir treatment for up to 5 years in patients with hepatitis B e antigen-positive chronic hepatitis B. Hepatology 51 (2), 422–430.
Chang, T.-T., Liaw, Y.-F. & Wu, S.-S. et al. (2010b) Long-term entecavir therapy results in the reversal of fibrosis/cirrhosis and continued histological improvement in patients with chronic hepatitis B. Hepatology 52 (3), 886–893.
Chen, C.-H., Lee, C.-M. & Lai, H.-C. et al. (2017) Prediction model of hepatocellular carcinoma risk in Asian patients with chronic hepatitis B treated with entecavir. Oncotarget 8 (54), 92431–92441.
Chen, C.-J., Yang, H.-I. & Su, J. et al. (2006) Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295 (1), 65–73.
Chen, J.-D., Yang, H.-I. & Iloeje, U. H. et al. (2010) Carriers of inactive hepatitis B virus are still at risk for hepatocellular carcinoma and liver-related death. Gastroenterology 138 (5), 1747–1754.
Choi, W.-M., Kim, G.-A. & Choi, J. et al. (2024) Non-linear association of baseline viral load with on-treatment hepatocellular carcinoma risk in chronic hepatitis B. Gut 73 (4), 649–658.
Choi, W.-M., Yip, T. C.-F. & Wong, G. L.-H. et al. (2023) Hepatocellular carcinoma risk in patients with chronic hepatitis B receiving tenofovir- vs. entecavir-based regimens: Individual patient data meta-analysis. Journal of hepatology 78 (3), 534–542.
Cornberg, M., Lok, A. S.-F., Terrault, N. A. & Zoulim, F. (2020) Guidance for design and endpoints of clinical trials in chronic hepatitis B - Report from the 2019 EASL-AASLD HBV Treatment Endpoints Conference‡. Journal of hepatology 72 (3), 539–557.
Cornberg, M., Sandmann, L. & Protzer, U. et al. (2021) S3 Guideline of the German Society for Gastroenterology, Digestive and Metabolic Diseases (DGVS) on the Prophylaxis, Diagnosis, and Treatment of Hepatitis B Virus Infection: S3-Leitlinie der Deutschen Gesellschaft für Gastroenterologie, Verdauungs- und Stoffwechselkrankheiten (DGVS) zur Prophylaxe, Diagnostik und Therapie der Hepatitis-B-Virusinfektion - (AWMF-Register-Nr. 021-11). Zeitschrift fur Gastroenterologie 59 (7), 691–776.
Craxì, A., Di Bona, D. & Cammà, C. (2003) Interferon-alpha for HBeAg-positive chronic hepatitis B. Journal of hepatology 39 Suppl 1, S99-105.
Da Wang, F., Zhou, J. & Li, L.-Q. et al. (2023) Improved bone and renal safety in younger tenofovir disoproxil fumarate experienced chronic hepatitis B patients after switching to tenofovir alafenamide or entecavir. Annals of hepatology 28 (5), 101119.
Dienstag, J. L., Perrillo, R. P., Schiff, E. R., Bartholomew, M., Vicary, C. & Rubin, M. (1995) A preliminary trial of lamivudine for chronic hepatitis B infection. The New England journal of medicine 333 (25), 1657–1661.
EASL (2017) Clinical Practice Guidelines on the management of hepatitis B virus infection. Journal of hepatology 67 (2), 370–398.
Fan, R., Papatheodoridis, G. & Sun, J. et al. (2020) aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. Journal of hepatology 73 (6), 1368–1378.
Feld, J. J., Lok, A. S. & Zoulim, F. (2023) New Perspectives on Development of Curative Strategies for Chronic Hepatitis B. Clinical gastroenterology and hepatology the official clinical practice journal of the American Gastroenterological Association 21 (8), 2040–2050.
Fleischer, R. D. & Lok, A. S. F. (2009) Myopathy and neuropathy associated with nucleos(t)ide analog therapy for hepatitis B. Journal of hepatology 51 (4), 787–791.
Flink, H. J., van Zonneveld, M., Hansen, B. E., Man, R. A. de, Schalm, S. W. & Janssen, H. L. A. (2006) Treatment with Peg-interferon alpha-2b for HBeAg-positive chronic hepatitis B: HBsAg loss is associated with HBV genotype. The American journal of gastroenterology 101 (2), 297–303.
Fung, S., Kwan, P. & Fabri, M. et al. (2014) Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 146 (4), 980–988.
Ghany, M. G., Feld, J. J. & Chang, K.-M. et al. (2020) Serum alanine aminotransferase flares in chronic hepatitis B infection: the good and the bad. The lancet. Gastroenterology & hepatology 5 (4), 406–417.
Gish, R. G., Chang, T.-T. & Lai, C.-L. et al. (2010) Loss of HBsAg antigen during treatment with entecavir or lamivudine in nucleoside-naïve HBeAg-positive patients with chronic hepatitis B. Journal of viral hepatitis 17 (1), 16–22.
Hadziyannis, S. J. & Papatheodoridis, G. V. (2006a) Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Seminars in liver disease 26 (2), 130–141.
Hadziyannis, S. J., Tassopoulos, N. C. & Heathcote, E. J. et al. (2006b) Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B for up to 5 years. Gastroenterology 131 (6), 1743–1751.
Hadziyannis, S. J. & Vassilopoulos, D. (2001) Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 34 (4 Pt 1), 617–624.
Heathcote, E. J., Marcellin, P. & Buti, M. et al. (2011) Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology 140 (1), 132–143.
Hosaka, T., Suzuki, F. & Kobayashi, M. et al. (2013) Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 58 (1), 98–107.
Hou, J., Yin, Y.-K. & Xu, D. et al. (2008) Telbivudine versus lamivudine in Chinese patients with chronic hepatitis B: Results at 1 year of a randomized, double-blind trial. Hepatology 47 (2), 447–454.
Hou, J.-L., Zhao, W. & Lee, C. et al. (2020) Outcomes of Long-term Treatment of Chronic HBV Infection With Entecavir or Other Agents From a Randomized Trial in 24 Countries. Clinical gastroenterology and hepatology the official clinical practice journal of the American Gastroenterological Association 18 (2), 457-467.e21.
Hsu, Y.-C., Yip, T. C.-F. & Ho, H. J. et al. (2018) Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. Journal of hepatology 69 (2), 278–285.
Huang, D. Q., Tamaki, N. & Lee, H. W. et al. (2023) Outcome of untreated low-level viremia versus antiviral therapy-induced or spontaneous undetectable HBV-DNA in compensated cirrhosis. Hepatology 77 (5), 1746–1756.
Huang, M., Lin, G. & Shi, H. et al. (2017) TDF Monotherapy Is Effective Regardless of Prior Nucleos(t)ide Analogue Treatment in Chronic Hepatitis B Patients in China. BioMed research international 2017, 2463197.
Iloeje, U. H., Yang, H.-I., Su, J., Jen, C.-L., You, S.-L. & Chen, C.-J. (2006) Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130 (3), 678–686.
Janssen, H. L. A., van Zonneveld, M. & Senturk, H. et al. (2005) Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet (London, England) 365 (9454), 123–129.
Jochum, C., Maischack, F. & Anastasiou, O. E. et al. (2016) Therapie der akuten fulminanten Hepatitis B mit Nucleos(t)id-Analogen ist sicher und führt nicht zur Chronifizierung der Hepatitis B. Zeitschrift fur Gastroenterologie 54 (12), 1306–1311.
KASL (2022) KASL clinical practice guidelines for management of chronic hepatitis B. Clinical and Molecular Hepatology 28 (2), 276–331.
Keeffe, E. B., Zeuzem, S. & Koff, R. S. et al. (2007) Report of an international workshop: Roadmap for management of patients receiving oral therapy for chronic hepatitis B. Clinical gastroenterology and hepatology the official clinical practice journal of the American Gastroenterological Association 5 (8), 890–897.
Kim, G.-A., Lim, Y.-S. & An, J. et al. (2014) HBsAg seroclearance after nucleoside analogue therapy in patients with chronic hepatitis B: clinical outcomes and durability. Gut 63 (8), 1325–1332.
Kumar, M., Satapathy, S. & Monga, R. et al. (2007) A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology 45 (1), 97–101.
Lai, C.-L., Gane, E. & Liaw, Y.-F. et al. (2007) Telbivudine versus lamivudine in patients with chronic hepatitis B. The New England journal of medicine 357 (25), 2576–2588.
Lai, C.-L., Leung, N. & Teo, E.-K. et al. (2005) A 1-year trial of telbivudine, lamivudine, and the combination in patients with hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 129 (2), 528–536.
Lampertico, P., Del Ninno, E. & Viganò, M. et al. (2003) Long-term suppression of hepatitis B e antigen-negative chronic hepatitis B by 24-month interferon therapy. Hepatology (Baltimore, Md.) 37 (4), 756–763.
Lampertico, P., Viganò, M. & Di Costanzo, G. G. et al. (2013) Randomised study comparing 48 and 96 weeks peginterferon α-2a therapy in genotype D HBeAg-negative chronic hepatitis B. Gut 62 (2), 290–298.
Lampertico, P., Viganò, M. & Facchetti, F. et al. (2010) 1009 EFFECTIVENESS OF ENTECAVIR FOR NUC-NAIVE, HBEAG-NEGATIVE CHRONIC HEPATITIS B PATIENTS IN CLINICAL PRACTICE: A 2-YEAR MULTICENTER COHORT STUDY IN 311 PATIENTS. Journal of hepatology 52, S389-S390.
Lange, C. M., Bojunga, J. & Hofmann, W. P. et al. (2009) Severe lactic acidosis during treatment of chronic hepatitis B with entecavir in patients with impaired liver function. Hepatology 50 (6), 2001–2006.
Lau, G., Yu, M.-L. & Wong, G. et al. (2021) APASL clinical practice guideline on hepatitis B reactivation related to the use of immunosuppressive therapy. Hepatology International 15 (5), 1031–1048.
Lau, G. K. K., Piratvisuth, T. & Luo, K. X. et al. (2005) Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B. The New England journal of medicine 352 (26), 2682–2695.
Lavanchy, D. (2004) Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. Journal of viral hepatitis 11 (2), 97–107.
Lee, H. W., Yoo, E. J. & Kim, B. K. et al. (2014) Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. The American journal of gastroenterology 109 (8), 1241–1249.
Lee, S. H., Cheon, G. J. & Kim, H. S. et al. (2018) Tenofovir disoproxil fumarate monotherapy is superior to entecavir-adefovir combination therapy in patients with suboptimal response to lamivudine-adefovir therapy for nucleoside-resistant HBV: a 96-week prospective multicentre trial. Antiviral therapy 23 (3), 219–227.
Liaw, Y.-F., Gane, E. & Leung, N. et al. (2009) 2-Year GLOBE trial results: telbivudine Is superior to lamivudine in patients with chronic hepatitis B. Gastroenterology 136 (2), 486–495.
Liaw, Y.-F., Jia, J.-D. & Chan, H. L. Y. et al. (2011) Shorter durations and lower doses of peginterferon alfa-2a are associated with inferior hepatitis B e antigen seroconversion rates in hepatitis B virus genotypes B or C. Hepatology 54 (5), 1591–1599.
Lim, Y.-S., Chan, H. L. Y. & Ahn, S. H. et al. (2023) Tenofovir alafenamide and tenofovir disoproxil fumarate reduce incidence of hepatocellular carcinoma in patients with chronic hepatitis B. JHEP reports innovation in hepatology 5 (10), 100847.
Liu, J., Li, T., Zhang, L. & Xu, A. (2019) The Role of Hepatitis B Surface Antigen in Nucleos(t)ide Analogues Cessation Among Asian Patients With Chronic Hepatitis B: A Systematic Review. Hepatology 70 (3), 1045–1055.
Lok, A. S., Trinh, H. & Carosi, G. et al. (2012) Efficacy of entecavir with or without tenofovir disoproxil fumarate for nucleos(t)ide-naïve patients with chronic hepatitis B. Gastroenterology 143 (3), 619-628.e1.
Lumley, S. F., Delphin, M. & Mokaya, J. F. et al. (2024) A systematic review and meta-analysis of the risk of hepatitis B virus (HBV) resistance in people treated with entecavir or tenofovir. Journal of clinical virology the official publication of the Pan American Society for Clinical Virology 174, 105711.
Manesis, E. K. & Hadziyannis, S. J. (2001) Interferon alpha treatment and retreatment of hepatitis B e antigen-negative chronic hepatitis B. Gastroenterology 121 (1), 101–109.
Manno, M., Cammà, C. & Schepis, F. et al. (2004) Natural history of chronic HBV carriers in northern Italy: morbidity and mortality after 30 years. Gastroenterology 127 (3), 756–763.
Marcellin, P., Ahn, S. H. & Ma, X. et al. (2016) Combination of Tenofovir Disoproxil Fumarate and Peginterferon α-2a Increases Loss of Hepatitis B Surface Antigen in Patients With Chronic Hepatitis B. Gastroenterology 150 (1), 134-144.e10.
Marcellin, P., Bonino, F. & Lau, G. K. K. et al. (2009) Sustained response of hepatitis B e antigen-negative patients 3 years after treatment with peginterferon alpha-2a. Gastroenterology 136 (7), 2169-2179.e1-4.
Marcellin, P., Chang, T.-T. & Lim, S. G. et al. (2003) Adefovir dipivoxil for the treatment of hepatitis B e antigen-positive chronic hepatitis B. The New England journal of medicine 348 (9), 808–816.
Marcellin, P., Gane, E. & Buti, M. et al. (2013) Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet (London, England) 381 (9865), 468–475.
Marcellin, P., Heathcote, J. E. & Buti, M. et al. (2011) Characterization of HBsAg Kinetics and HBsAg Seroconversion in Patients with Chronic Hepatitis B (CHB) Treated with Tenofovir Disoproxil Fumarate (TDF).
Marcellin, P., Lau, G. K. K. & Bonino, F. et al. (2004) Peginterferon alfa-2a alone, lamivudine alone, and the two in combination in patients with HBeAg-negative chronic hepatitis B. The New England journal of medicine 351 (12), 1206–1217.
Marcellin, P., Wursthorn, K. & Wedemeyer, H. et al. (2015) Telbivudine plus pegylated interferon alfa-2a in a randomized study in chronic hepatitis B is associated with an unexpected high rate of peripheral neuropathy. Journal of hepatology 62 (1), 41–47.
Martinot-Peignoux, M., Lapalus, M. & Laouénan, C. et al. (2013) Prediction of disease reactivation in asymptomatic hepatitis B e antigen-negative chronic hepatitis B patients using baseline serum measurements of HBsAg and HBV-DNA. Journal of clinical virology the official publication of the Pan American Society for Clinical Virology 58 (2), 401–407.
McMahon, B. J., Alward, W. L. & Hall, D. B. et al. (1985) Acute hepatitis B virus infection: relation of age to the clinical expression of disease and subsequent development of the carrier state. The Journal of infectious diseases 151 (4), 599–603.
Moini, M. & Fung, S. (2022) HBsAg Loss as a Treatment Endpoint for Chronic HBV Infection: HBV Cure. Viruses 14 (4).
Mommeja-Marin, H., Mondou, E., Blum, M. R. & Rousseau, F. (2003) Serum HBV DNA as a marker of efficacy during therapy for chronic HBV infection: analysis and review of the literature. Hepatology (Baltimore, Md.) 37 (6), 1309–1319.
Morais, E., Mason, L. & Dever, J. et al. (2023) Clinical Consequences of Hepatitis B Surface Antigen Loss in Chronic Hepatitis B Infection: A Systematic Literature Review and Meta-Analysis. Gastro hep advances 2 (7), 992–1004.
Moses, S. E., Lim, Z. Y. & Sudhanva, M. et al. (2006) Lamivudine prophylaxis and treatment of hepatitis B Virus-exposed recipients receiving reduced intensity conditioning hematopoietic stem cell transplants with alemtuzumab. Journal of medical virology 78 (12), 1560–1563.
Moucari, R., Korevaar, A. & Lada, O. et al. (2009) High rates of HBsAg seroconversion in HBeAg-positive chronic hepatitis B patients responding to interferon: a long-term follow-up study. Journal of hepatology 50 (6), 1084–1092.
Ning, Q., Han, M. & Sun, Y. et al. (2014) Switching from entecavir to PegIFN alfa-2a in patients with HBeAg-positive chronic hepatitis B: a randomised open-label trial (OSST trial). Journal of hepatology 61 (4), 777–784.
Pan, C. Q., Zhu, L., Yu, A. S., Zhao, Y., Zhu, B. & Dai, E. (2024a) Tenofovir Alafenamide versus Tenofovir Disoproxil Fumarate for Preventing Vertical Transmission in Chronic Hepatitis B Mothers: A Systematic Review and Meta-Analysis. Clinical infectious diseases an official publication of the Infectious Diseases Society of America.
Pan, S., Zhang, Y., Zeng, Y. & Lin, C. (2024b) Comparison of the efficacy and safety of TAF, TDF, and LdT to prevent the transmission of hepatitis B in pregnant women: A retrospective study. Immunity, inflammation and disease 12 (2), e1204.
Papatheodoridis, G., Dalekos, G. & Sypsa, V. et al. (2016a) PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. Journal of hepatology 64 (4), 800–806.
Papatheodoridis, G., Vlachogiannakos, I. & Cholongitas, E. et al. (2016b) Discontinuation of oral antivirals in chronic hepatitis B: A systematic review. Hepatology 63 (5), 1481–1492.
Papatheodoridis, G. V., Chan, H. L.-Y., Hansen, B. E., Janssen, H. L. A. & Lampertico, P. (2015) Risk of hepatocellular carcinoma in chronic hepatitis B: assessment and modification with current antiviral therapy. Journal of hepatology 62 (4), 956–967.
Papatheodoridis, G. V., Idilman, R. & Dalekos, G. N. et al. (2017) The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 66 (5), 1444–1453.
Papatheodoridis, G. V., Sypsa, V. & Dalekos, G. et al. (2018) Eight-year survival in chronic hepatitis B patients under long-term entecavir or tenofovir therapy is similar to the general population. Journal of hepatology 68 (6), 1129–1136.
Perrillo, R. P., Lai, C.-L. & Liaw, Y.-F. et al. (2002) Predictors of HBeAg loss after lamivudine treatment for chronic hepatitis B. Hepatology (Baltimore, Md.) 36 (1), 186–194.
Petersen, J., Ratziu, V. & Buti, M. et al. (2012) Entecavir plus tenofovir combination as rescue therapy in pre-treated chronic hepatitis B patients: an international multicenter cohort study. Journal of hepatology 56 (3), 520–526.
Rehermann, B., Ferrari, C., Pasquinelli, C. & Chisari, F. V. (1996) The hepatitis B virus persists for decades after patients' recovery from acute viral hepatitis despite active maintenance of a cytotoxic T-lymphocyte response. Nature medicine 2 (10), 1104–1108.
Revill, P. A., Chisari, F. V. & Block, j. M. et al. (2019) A global scientific strategy to cure hepatitis B. The lancet. Gastroenterology & hepatology 4 (7), 545–558.
Roche, B. & Samuel, D. (2011) The difficulties of managing severe hepatitis B virus reactivation. Liver international official journal of the International Association for the Study of the Liver 31 Suppl 1, 104–110.
Sarin, S. K., Kumar, M. & Lau, G. K. et al. (2016) Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatology International 10 (1), 1–98.
Schiff, E. R., Lee, S. S. & Chao, Y.-C. et al. (2011) Long-term treatment with entecavir induces reversal of advanced fibrosis or cirrhosis in patients with chronic hepatitis B. Clinical gastroenterology and hepatology the official clinical practice journal of the American Gastroenterological Association 9 (3), 274–276.
Seto, W.-K., Liu, K. S. & Mak, L.-Y. et al. (2021) Role of serum HBV RNA and hepatitis B surface antigen levels in identifying Asian patients with chronic hepatitis B suitable for entecavir cessation. Gut 70 (4), 775–783.
Sherman, M., Yurdaydin, C. & Simsek, H. et al. (2008) Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology 48 (1), 99–108.
Siberry, G. K., Jacobson, D. L. & Kalkwarf, H. J. et al. (2015) Lower Newborn Bone Mineral Content Associated With Maternal Use of Tenofovir Disoproxil Fumarate During Pregnancy. Clinical infectious diseases an official publication of the Infectious Diseases Society of America 61 (6), 996–1003.
Sohn, W., Cho, J.-Y. & Kim, J. H. et al. (2017) Risk score model for the development of hepatocellular carcinoma in treatment-naïve patients receiving oral antiviral treatment for chronic hepatitis B. Clinical and Molecular Hepatology 23 (2), 170–178.
Sonneveld, M. J., Rijckborst, V., Boucher, C. A. B., Hansen, B. E. & Janssen, H. L. A. (2010) Prediction of sustained response to peginterferon alfa-2b for hepatitis B e antigen-positive chronic hepatitis B using on-treatment hepatitis B surface antigen decline. Hepatology 52 (4), 1251–1257.
Su, T.-H., Hu, T.-H. & Chen, C.-Y. et al. (2016) Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver international official journal of the International Association for the Study of the Liver 36 (12), 1755–1764.
Sung, J. J. Y., Lai, J.-Y. & Zeuzem, S. et al. (2008) Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. Journal of hepatology 48 (5), 728–735.
Tabak, F., Yurdaydın, C. & Kaymakoğlu, S. et al. (2017) Diagnosis, management and treatment of hepatitis B virus infection: Turkey 2017 Clinical Practice Guidelines. The Turkish Journal of Gastroenterology 28 (Suppl 2), 73–83.
Tassopoulos, N. C., Papaevangelou, G. J., Sjogren, M. H., Roumeliotou-Karayannis, A., Gerin, J. L. & Purcell, R. H. (1987) Natural history of acute hepatitis B surface antigen-positive hepatitis in Greek adults. Gastroenterology 92 (6), 1844–1850.
Tenney, D. J., Rose, R. E. & Baldick, C. J. et al. (2009) Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naïve patients is rare through 5 years of therapy. Hepatology 49 (5), 1503–1514.
Terrault, N. A., Lok, A. S. F. & McMahon, B. J. et al. (2018) Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 67 (4), 1560–1599.
Testoni, B., Scholtès, C. & Plissonnier, M.-L. et al. (2024) Quantification of circulating HBV RNA expressed from intrahepatic cccDNA in untreated and NUC treated patients with chronic hepatitis B. Gut 73 (4), 659–667.
Tillmann, H. L., Hadem, J. & Leifeld, L. et al. (2006) Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. Journal of viral hepatitis 13 (4), 256–263.
Tout, I., Lampertico, P., Berg, T. & Asselah, T. (2021) Perspectives on stopping nucleos(t)ide analogues therapy in patients with chronic hepatitis B. Antiviral research 185, 104992.
Toy, M., Veldhuijzen, I. K., Man, R. A. de, Richardus, J. H. & Schalm, S. W. (2009) Potential impact of long-term nucleoside therapy on the mortality and morbidity of active chronic hepatitis B. Hepatology 50 (3), 743–751.
Tseng, T.-C., Chiang, C. & Liu, C.-J. et al. (2023) Low Hepatitis B Core-Related Antigen Levels Correlate Higher Spontaneous Seroclearance of Hepatitis B Surface Antigen in Chronic Hepatitis B Patients With High Hepatitis B Surface Antigen Levels. Gastroenterology 164 (4), 669-679.e6.
Tseng, T.-C., Liu, C.-J. & Yang, H.-C. et al. (2013) Serum hepatitis B surface antigen levels help predict disease progression in patients with low hepatitis B virus loads. Hepatology 57 (2), 441–450.
van Bömmel, F. & Berg, T. (2018) Stopping long-term treatment with nucleos(t)ide analogues is a favourable option for selected patients with HBeAg-negative chronic hepatitis B. Liver international official journal of the International Association for the Study of the Liver 38 Suppl 1, 90–96.
van Bömmel, F., Man, R. A. de & Wedemeyer, H. et al. (2010) Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology 51 (1), 73–80.
van Bömmel, F., Stein, K. & Heyne, R. et al. (2023) A multicenter randomized-controlled trial of nucleos(t)ide analogue cessation in HBeAg-negative chronic hepatitis B. Journal of hepatology 78 (5), 926–936.
van Bömmel, F., Trojan, J. & Deterding, K. et al. (2012) Evolution of adefovir-resistant HBV polymerase gene variants after switching to tenofovir disoproxil fumarate monotherapy. Antiviral therapy 17 (6), 1049–1058.
van Hees, S., Bourgeois, S. & van Vlierberghe, H. et al. (2018) Stopping nucleos(t)ide analogue treatment in Caucasian hepatitis B patients after HBeAg seroconversion is associated with high relapse rates and fatal outcomes. Alimentary pharmacology & therapeutics 47 (8), 1170–1180.
van Zonneveld, M., van Nunen, A. B., Niesters, H. G. M., Man, R. A. de, Schalm, S. W. & Janssen, H. L. A. (2003) Lamivudine treatment during pregnancy to prevent perinatal transmission of hepatitis B virus infection. Journal of viral hepatitis 10 (4), 294–297.
Vassilopoulos, D. & Calabrese, L. H. (2007) Risks of immunosuppressive therapies including biologic agents in patients with rheumatic diseases and co-existing chronic viral infections. Current opinion in rheumatology 19 (6), 619–625.
Veronese, P., Dodi, I., Esposito, S. & Indolfi, G. (2021) Prevention of vertical transmission of hepatitis B virus infection. World journal of gastroenterology 27 (26), 4182–4193.
Wang, J., Shen, T. & Huang, X. et al. (2016) Serum hepatitis B virus RNA is encapsidated pregenome RNA that may be associated with persistence of viral infection and rebound. Journal of hepatology 65 (4), 700–710.
Werle-Lapostolle, B., Bowden, S. & Locarnini, S. et al. (2004) Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology 126 (7), 1750–1758.
WHO (2024) Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis B infection.
Woldemedihn, G. M., Aberra, H. & Desalegn, H. et al. (2023) Renal Safety of Long-term Tenofovir Disoproxil Fumarate Treatment in Patients With Chronic Hepatitis B. Open Forum Infectious Diseases 10 (8), ofad404.
Wong, D. K., Cheung, A. M., O'Rourke, K., Naylor, C. D., Detsky, A. S. & Heathcote, J. (1993) Effect of alpha-interferon treatment in patients with hepatitis B e antigen-positive chronic hepatitis B. A meta-analysis. Annals of internal medicine 119 (4), 312–323.
Wu, C.-Y., Lin, J.-T. & Ho, H. J. et al. (2014) Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: a nationwide cohort study. Gastroenterology 147 (1), 143-151.e5.
Xing, T., Xu, H., Cao, L. & Ye, M. (2017) HBeAg Seroconversion in HBeAg-Positive Chronic Hepatitis B Patients Receiving Long-Term Nucleos(t)ide Analog Treatment: A Systematic Review and Network Meta-Analysis. PLoS ONE 12 (1), e0169444.
Xu, X., Jiang, L., Zeng, Y., Pan, L., Lou, Z. & Ruan, B. (2023) HCC prediction models in chronic hepatitis B patients receiving entecavir or tenofovir: a systematic review and meta-analysis. Virology Journal 20 (1), 180.
Yang, H., Bae, S. H. & Nam, H. et al. (2022) A risk prediction model for hepatocellular carcinoma after hepatitis B surface antigen seroclearance. Journal of hepatology 77 (3), 632–641.
Yip, T. C.-F., Lai, J. C.-T. & Yam, T.-F. et al. (2024) Long-term use of tenofovir disoproxil fumarate increases fracture risk in elderly patients with chronic hepatitis B. Journal of hepatology 80 (4), 553–563.
Yip, T. C.-F., Wong, G. L.-H. & Chan, H. L.-Y. et al. (2019) HBsAg seroclearance further reduces hepatocellular carcinoma risk after complete viral suppression with nucleos(t)ide analogues. Journal of hepatology 70 (3), 361–370.
Yu, J.-W., Sun, L.-J., Zhao, Y.-H., Kang, P. & Li, S.-C. (2010) The study of efficacy of lamivudine in patients with severe acute hepatitis B. Digestive diseases and sciences 55 (3), 775–783.
Yuen, M.-F., Fong, D. Y.-T., Wong, D. K.-H., Yuen, J. C.-H., Fung, J. & Lai, C.-L. (2007) Hepatitis B virus DNA levels at week 4 of lamivudine treatment predict the 5-year ideal response. Hepatology 46 (6), 1695–1703.
Zeuzem, S., Gane, E. & Liaw, Y.-F. et al. (2009) Baseline characteristics and early on-treatment response predict the outcomes of 2 years of telbivudine treatment of chronic hepatitis B. Journal of hepatology 51 (1), 11–20.
Zoulim, F. & Locarnini, S. (2012) Management of treatment failure in chronic hepatitis B. Journal of hepatology 56 Suppl 1, S112-22.
