10. Hepatitis D – diagnosis and treatment
Heiner Wedemeyer
Introduction
Hepatitis delta is the most severe form of viral hepatitis in humans. The hepatitis delta virus (HDV) is a defective RNA virus which requires the hepatitis B virus (HBV) surface antigen (HBsAg) for complete replication and transmission, while the full extent of the HBV helper function is unexplored (Rizzetto 1983, Taylor 2012). Hence, HDV occurs only in HBsAg positive individuals either as acute coinfection or as superinfection in patients with chronic HBV (Wedemeyer 2010) (Figure 1). Several studies have shown that chronic HDV infection leads to more severe liver disease than chronic HBV monoinfection, with an accelerated course of fibrosis progression, possibly a slightly increased risk of hepatocellular carcinoma and early decompensation in the setting of established cirrhosis (Hughes 2011, Manesis 2013, Beguelin 2017). Simultaneous HBV and HDV infection has also been shown to be more severe than infection with HBV alone in chimpanzees (Dienes 1990). An easy to apply clinical score has been suggested to predict the likelihood of experiencing a clinical event for patients with HDV, the baseline-event-anticipation (BEA) score (Calle-Serrano 2014). So far, only interferon α treatment is recommended against HDV (Deterding 2019) and has been linked to improve the clinical long-term outcome (Farci 2004, Wranke 2017, Yurdaydin 2018). Data on the use of pegylated interferon (PEG-IFN) confirm earlier findings, leading to prolonged virological off-treatment responses in about one quarter of patients but long-term HDV RNA relapses may occur (Heidrich 2014). Thus, HBsAg clearance should be the preferred endpoint of interferon-based therapies of HDV. Still, suppression of HDV RNA in the presence of HBsAg has been associated with improved clinical outcomes. Alternative treatment options including HBV entry inhibitors and prenylation inhibitors (www.clinicaltrials.gov) are currently in phase 3 clinical development.
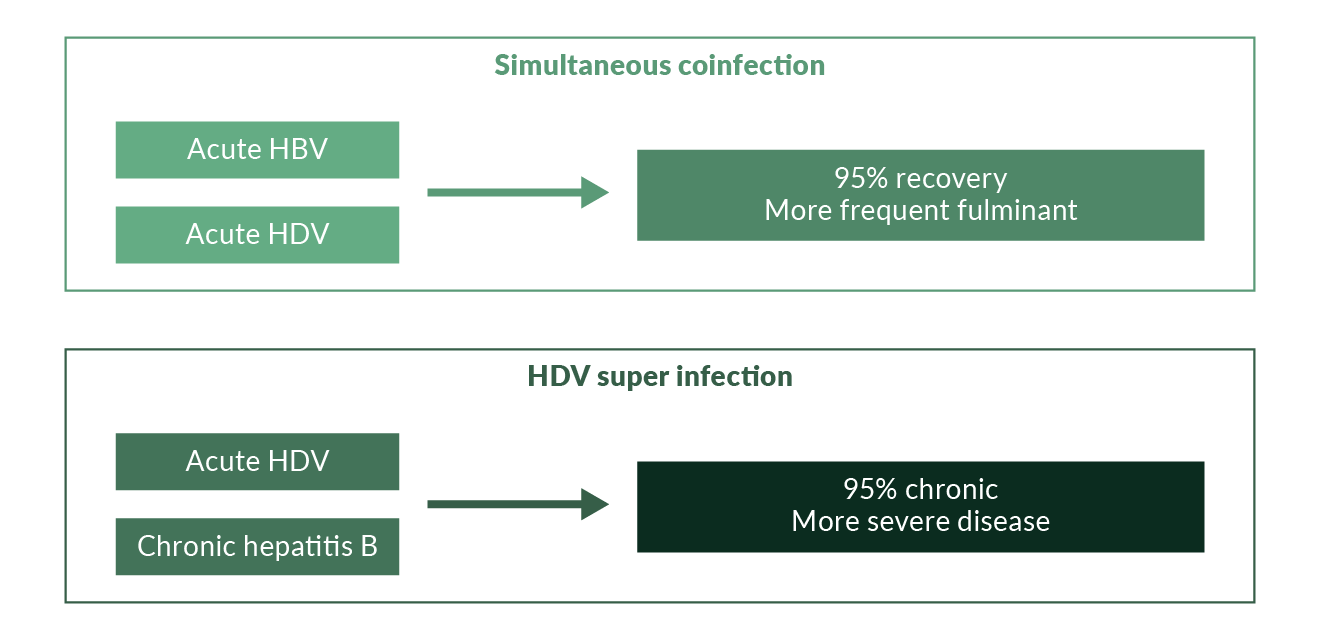 Figure 1. Courses of hepatitis delta
Figure 1. Courses of hepatitis delta
Virology of HDV
The hepatitis D virion is approximately 36 nm in size, containing HDV RNA and delta antigen. HDV RNA is single-stranded, highly base-paired, circular and by far the smallest known genome of any animal virus, containing close to 1700 nucleotides (Taylor 2012, Sureau 2016). It is coated with the envelope protein derived from the pre-S and S antigens of HBV. Other enveloped viruses including HCV and VSV can also propagate HDV infection, both in vitro as well in humanized mice (Peres-Vargas 2019). Still, it is currently unclear if viruses distinct from HBV induce dissemination of HDV also in patients. The HDV RNA has six open reading frames (ORFs), three on the genomic and three on the antigenomic strand. One ORF codes for the hepatitis delta antigen (HDAg), while the other ORFs do not appear to be actively transcribed. Two HDAgs exist: the small HDAg (24 kD) is 155 amino acids long and the large HDAg (27 kD) is 214 amino acids long. A single nucleotide change (A-G) in the small HDAg sequence leads to the synthesis of the large HDAg. The small HDAg accelerates genome synthesis, while the large HDAg that inhibits HDV RNA synthesis is necessary for virion morphogenesis (Taylor 2012). Replication of HDV RNA occurs through a ‘double rolling circle’ model in which the genomic strand is replicated by a host RNA polymerase to yield a multimeric linear structure that is then autocatalytically cleaved to linear monomers and ligated into the circular HDV RNA viral progeny (Sureau 2016). Recent work showed that the host RNA polymerase II-is coactivated by S-HDAg using a histone mimicry strategy (Abeywickrama-Samarakoon 2020).
Genetic analysis has revealed the presence of at least eight HDV genotypes (Le Gal 2017) (Figure 2). Genotype 1 is the most frequently seen and is distributed throughout the world, especially in Europe, the Middle East, North America and North Africa. Genotype 2 is seen in East Asia and the Yakutia region of Russia, and genotype 3 is seen exclusively in the northern part of South America, especially in the Amazon basin. Genotype 4 is seen in Taiwan and Japan while genotypes 5–8 are found in Africa (Deny 2006). HDV genotype 1 is associated with both severe and mild disease whereas genotype 2 causes a milder disease over a long-term course (Su 2006). HDV genotype 5 may also take a milder course and a better response to PEG-IFNa treatment compared to genotype 1 (Spaan 2020).
HDV quasispecies evolution declines over time during HDV infection even though a continuous adaptation of HDV occurs indicating ongoing immune pressure in chronic HDV (Homs 2016).
HBV genotypes may also contribute to distinct clinical courses of HDV. There is no evidence that specific HDV genotypes may infect patients with one specific HBV genotype exclusively. However, recent data indicate that distinct HDV mutations may facilitate association of certain HDV genotypes with different HBV genotypes (Kay 2014). The global distribution of HBV and HDV genotypes is shown in Table 1.
Table 1. HBV and HDV genotypes| Region | HDV genotype | HBV genotype |
| Europe | 1 | D/A |
| Brazil | 1/3 | F/A/D |
| China, Taiwan, Japan | 1/2/4 | B/C |
| Turkey, Iran, Pakistan, India | 1 | D |
| Western Pacific | 1/2 | B/C/D |
| Africa | 1, 5–8 | D/A/E |
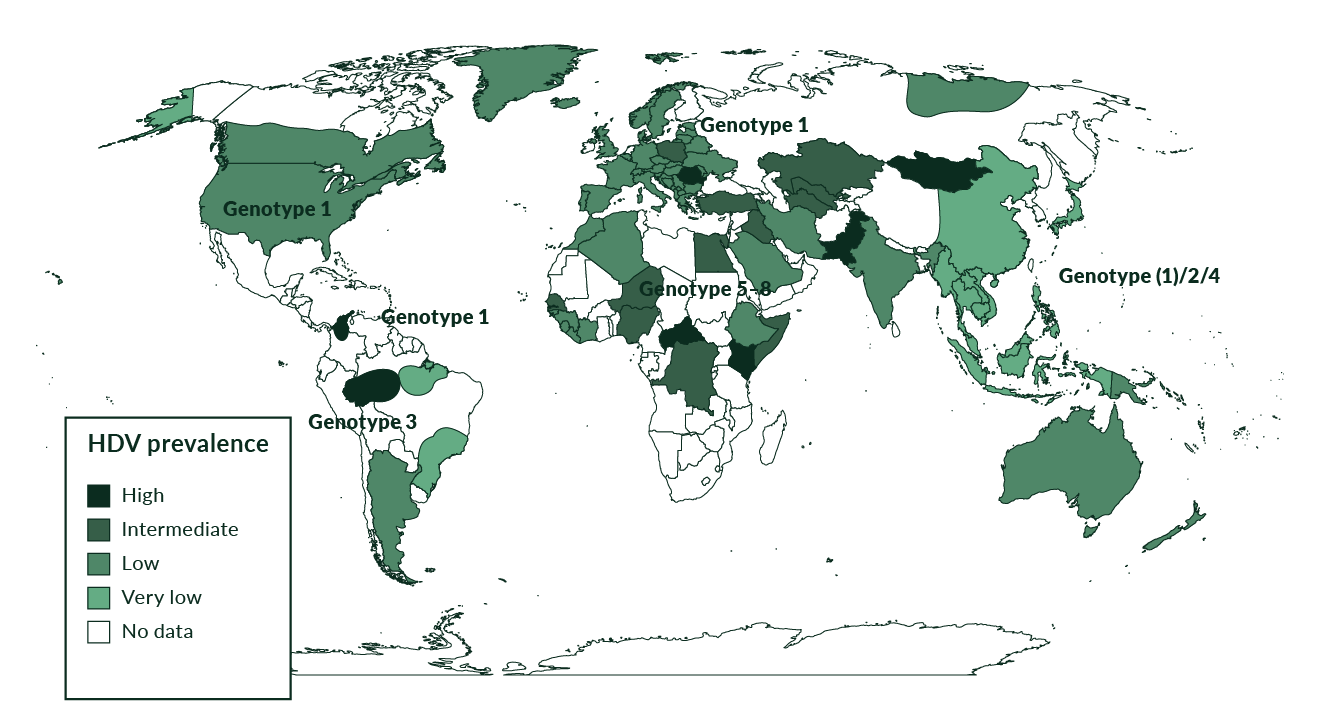 Figure 2. Prevalence of HDV genotypes
Figure 2. Prevalence of HDV genotypes
Epidemiology of HDV
HDV is not an uncommon disease. Being linked to HBV, HDV is spread in the same way as HBV, mainly through parenteral exposure (Niro 1999). It is highly endemic in Mediterranean countries, the Middle East, Central Africa, and northern parts of South America (Hughes 2011) (Figure 2). In high-income countries, high anti-HDV prevalence is found in people who inject drugs (PWID) who are HBsAg positive, both in Europe (Gaeta 2000, Heidrich 2009, Erhardt 2010) and North America (Kurcirka 2010). Worldwide, more than 240 million people are chronically infected with HBV and 15–25 million of those are estimated to be anti-HDV positive (Wedemeyer 2010). Two systematic reviews suggested that the prevalence of HDV may be even higher reaching up to 1% of the populations world-wide (Chen 2018, Miao 2019). This work has been criticized as systematic reviews can only be as good as studies included (Wedemeyer 2018). but HDV may still be more frequent than previously thought. HDV was endemic in Southern Europe. Several studies performed in the 1980s and 1990s showed a prevalence of anti-HDV of more than 20% among HBsAg positive individuals. As a result of the implementation of HBV vaccination programmes, the incidence of HDV infections significantly decreased in Southern Europe in the 1990s (Gaeta 2000, Degertekin 2008) (Figure 3). Other countries with a particularly high prevalence of HDV are Mongolia with up to one third of chronic hepatitis cases being caused by HDV (Tsatsralt-Od 2005), some Central Asian republics, Pakistan (Abbas 2012), northwestern states of Brazil (Kay 2014, Braga 2014), distinct regions in Africa (Andernach 2014), and some Polynesian islands (Han 2014). Of note, prevalence rates of HBV and HDV are not linked - for example, HDV infections have been considered to be rather rare in most parts of mainland China despite very high frequencies of HBV. However, some studies revealed an HDV prevalence of up to 6.5%, suggesting that HDV may be more frequent in China than previously thought (Lia 2014). HBV/HDV coinfection was also associated with higher frequencies of end-stage liver disease in that study. In Taiwan, a country with a well-established national HBV vaccination program, the epidemiology of HDV changed over the last 20 years with PWID and HIV positive persons being particular risk groups and representing a main reservoir for HDV infection (Hung 2014, Lin 2015, Lee 2015).
One problem is that many HBsAg positive patients are not tested for HDV. A study from Greece even suggests that HDV testing declined over the last 10 years and only about one-third of people with HBV are currently assessed for the presence of HDV antibodies (Manesis 2013). Similarly, the HDV testing rate was low in four hospitals in London where people with HDV frequently had severe disease and patients were of very diverse ethnicity (El Bouzidi 2015). In the United States Veterans Affairs medical system, only 8.5% of more than 25,000 HBsAg positive patients were tested for HDV. Of those, 3.4% had evidence for HDV and HDV was associated with a 2.9 fold higher HCC incidence and a higher risk of all-cause mortality (Kushner 2015).
In our experience at a referral centre for liver disease, about 8–10% of HBsAg positive patients test positive for anti-HDV as chronic HDV still represents a significant health burden in Central Europe, which is a source of immigration (Wedemeyer 2007, Heidrich 2009, Erhardt 2003, Erhardt 2010) (Figure 3, Table 1). More than three quarters of these HDV patients were not born in Germany. However, the geographical origin of our patients has changed during the last decade. While until the mid-1990s the majority of HDV positive patients were born in Turkey, the proportion of Eastern European patients has significantly increased in recent years (Wedemeyer 2007). Similarly, high HDV prevalence in immigrant populations has been described in clinics in the UK (Cross 2008), France and Italy (Le Gal 2007, Mele 2007). HDV can also be found in high frequencies in people living with HIV who are also HBsAg positive with about 14.6% in different European regions (Soriano 2011). In France, the prevalence of HDV infection has increased during the last 15 years, again mainly in pre-infected newly arriving immigrants (Servant-Delmas 2013).
HDV prevalence is much lower in HBV patients without specific risk factors and cohorts excluding a referral bias. In this setting, less than 1–2% of HBsAg positive individuals test anti-HDV positive, even in countries like Italy where HDV prevalence is thought to be higher than in Northern Europe (Ippolito 2011). Thus, even though HDV is a major problem in distinct regions and specific cohorts, HDV is overall a rare disease and has therefore been granted orphan designation both by the FDA and by the European Commission.
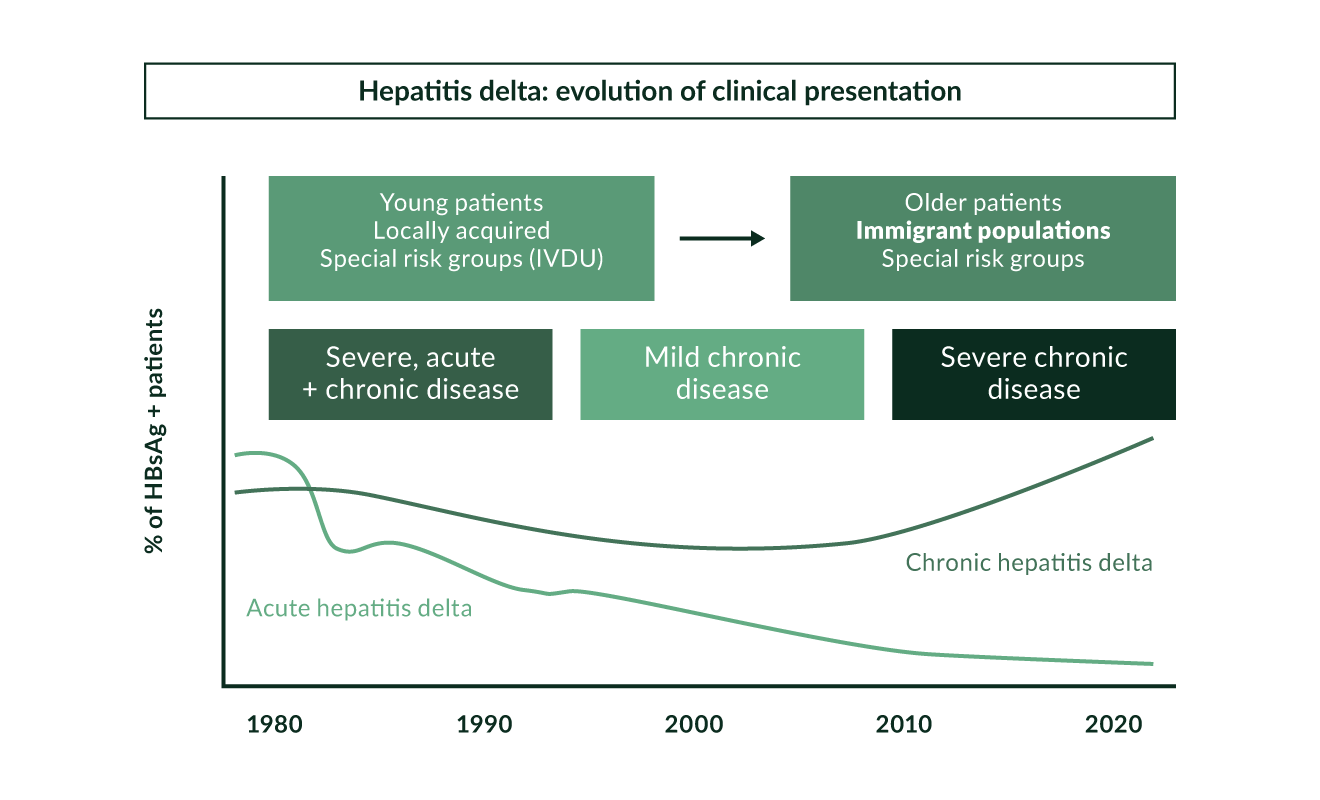 Figure 3. Hepatitis delta: evolution of clinical presentation
Figure 3. Hepatitis delta: evolution of clinical presentation
Pathogenesis of HDV
Knowledge about the pathogenesis of HDV infection is limited. Clinical observations have provided examples of mostly an immune-mediated process in HDV (Lunemann 2010). However, patterns suggesting a cytopathic viral disease have occasionally been observed. A typical example of this were outbreaks of severe hepatitis in the northern part of South America (Nakano 2001). These mostly fulminant hepatitis cases were induced by genotype 3 HDV. In HDV, liver histology is not different from a patient with HBV or HCV with accompanying necroinflammatory lesions. Importantly, HDV viraemia is not directly associated with the stage of liver disease in HDV genotype 1 infection (Zachou 2010) while in HDV genotype 3 infection higher viral loads were observed in patients with cirrhosis (Braga 2014). In both humanised chimeric mice as well as mice expressing the human HBV receptor (sodium taurocholate co-transporting polypeptide (NTCP)) HDV infection provoked a marked and broad induction of interferon stimulated genes and cytokines which was more pronounced than in HBV monoinfection (Giersch 2015, He 2015) which may directly contribute to the more severe inflammation in patients with HDV. Another study showed that modification of three amino acids in mouse NTCP (H84R, T86K, and S87N) rendered mice susceptible to HDV (He 2016). In this respect it is important to note that distinct polymorphisms in the IL28B gene may be associated with HBsAg persistence also in HDV coinfected patients (Karatayli 2015).
Cellular immune responses against the HDV have been described (Nisini 1997, Huang 2004, Grabowski 2011) suggesting that the quantity and quality of T cell responses may be associated with some control of the infection. The frequency of cytotoxic CD4+ T cells is higher in HDV patients than in individuals with HBV or HCV (Aslan 2006) and HDV-specific IFN gamma and IL-2 responses are more frequent in patients with low HDV viraemia (Grabowski 2011). Still, HDV-specific T cell responses are very weak in chronic infection. In vitro, the third signal cytokine IL-12 was able to restore the function of HDV-specific CD4+ and CD8+ T cells (Schirdewahn 2017). Still, HDV-specific T cells have been shown to induce immune pressure on HDV during leading to the development of T cell escape variants (Karimzadeh 2018; Karimzadeh 2019; Kefalakes 2019). However, in another study the breadth of HDV-specific T cell responses was not associated with the HDV replication status in patients (Landahl 2019). NK cells from patients with HDV have recently been investigated in more detail in comparison with other viral hepatitis infections (Lunemann 2014). Overall, NK cell frequencies increased but the cells were less activated and functionally impaired. HDV infection also did not alter NK cell differentiation, and the activity of liver disease reflected alterations in NK cell surface receptor expression. NK cell frequency may also be associated with early virological response to PEG-IFN α therapy although NK cells are severely functionally impaired during antiviral therapy (Lunemann 2015). Finally, mucosa-associated invariant T (MAIT) cells, which are innate-like T cells highly enriched in the human liver, are activated, functionally impaired and severely depleted in patients with chronic hepatitis D (Dias 2019). This loss of MAIT cells was associated with severity of liver disease. Collectively, this information suggests that HDV is mainly an immune-mediated disease, at least in HDV genotype 1 infection. Ideally, antiviral therapies should therefore also aim to enhance anti-HDV immunity to confer long-term control of the infection. Of note, chimpanzees that have recovered from HDV were successfully reinfected with HDV in one study performed in the 1980s (Negro 1988).
Coinfections with multiple hepatitis viruses are associated with diverse patterns of reciprocal inhibition of viral replication (Raimondo 2006, Wedemeyer 2010). HDV has frequently been shown to suppress HBV replication (Calle Serrano 2012). Between 70% and 90% of HDV patients are HBeAg negative with low levels of HBV DNA. Humanised HBsAg positive mice that become superinfected with HDV also show a decrease in HBV replication (Lütgehetmann 2012). A molecular explanation for the suppression of HBV replication by HDV has been suggested via the HDV proteins p24 and p27 repressing HBV enhancers (Williams 2009). In addition, induction of a type-I interferon response by HDV may contribute to HBV repression. This hypothesis is supported by the induction of interferon stimulated genes in HBV cells which were superinfected with HDV which led to a decrease of HBV replication markers (Alfaiate 2016). Viral dominance may change over time (Wedemeyer 2010) and about half of the hepatitis delta patients showed significant HBV replication in one study (Schaper 2010). A recent study from Brazil reported similar viral loads for HBV and HDV in 40% of patients infected with HDV genotype 3 and HDV dominance in 56% (Braga 2014). HDV may facilitate the selection of distinct HBV mutants which can have major implications for the replicative capacity of both viruses (Shirvani-Dastgerdi 2016). HDV entry into hepatocytes via NTCP may also be altered by the bile acid pool (Yan 2014, Veloso Alves Pereira 2015) even though administration of chenodeoxycolic acid to three chronically infected patients did not lead to a change in serum HDV RNA levels (Veloso Alves Pereira 2015).
There is increasing evidence that HDV not only suppresses HBV replication but also HCV replication in triple-infected patients. In our experience, less than one fifth of anti-HCV/HBsAg/anti-HDV positive individuals are positive for HCV RNA (Heidrich 2009). We even observed a case where acute HBV/HDV superinfection led to clearance of chronic HCV infection (Deterding 2009). It is not clear how many anti-HCV positive/HCV RNA negative patients have recovered from HCV infection and how many of these patients just show a suppressed HCV replication in the context of viral coinfections. Repeated HCV RNA testing is suggested in this context. We did not observe HCV relapses after interferon-induced cure of HDV (Wedemeyer 2011).
HDV may also play a direct role in the development of hepatocellular carcinoma by altering DNA methylation events (Benegiamo 2013). However, to what extent HDV infection is associated with an increased HCC risk is a matter of debate. Even though liver cancer can be found more frequently in patients with HDV (Manesis 2013, Romeo 2014), this may be explained by earlier development of liver cirrhosis and may not necessarily be a consequence of the direct oncogenic effects of HDV.
HDV might also have a role in other autoimmune diseases. HDV has been detected in salivary gland tissue of patients with Sjögren’s syndrome (SS) in the absence of HBsAg (Weller 2016). Interestingly, the expression of HDV antigens in salivary glands in mice resulted in the development of a SS-like phenotype. These findings have to be confirmed by others and it would be also interesting to investigate if HDV may cause similar pathology in other tissues.
Clinical course of HDV
Acute HBV/HDV coinfection
Acute HBV/HDV coinfection in adults leads to recovery in more than 90% of cases but frequently causes severe acute hepatitis with a high risk for developing a fulminant course (Rizzetto 2009). In contrast, HDV is cleared spontaneously only in a minority of patients with HDV superinfection of chronic HBsAg carriers (Figure 1). The observation that the histopathology of simultaneous HBV and HDV infection is more severe than in infection with HBV alone has also been documented in experiments with chimpanzees (Dienes 1990). Several outbreaks of very severe courses of acute HDV have been described in different regions of the world (Casey 1996, Flodgren 2000, Tsatsralt-Od 2006). Fortunately, acute HDV has become infrequent over the last two decades in high-income countries due to the introduction of vaccination programs (Figure 3).
Chronic HDV
Several early studies showed that chronic HDV leads to more severe liver disease compared to chronic HBV monoinfection, with an accelerated course of fibrosis progression, and early decompensation in the presence of cirrhosis (Farci 2012). HDV accounts for almost half of all cases of liver cirrhosis and hepatocellular carcinoma in southeast Turkey (Degertekin 2008). An observational study from Taiwan reported a cumulative survival of patients with HDV genotype 1 of as low as 50% after 15 years (Su 2006). Long-term follow-up data from Italy, Spain, Greece and Germany confirmed the particularly severe course of HDV (Romeo 2009, Niro 2010, Butí 2011, Manesis 2013, Calle-Serrano 2014). Characteristics of patients with HDV genotype 3 infection were reported in more detail recently (Braga 2014) confirming the severity of liver disease also for this specific HDV genotype. HDV infection has been associated with a particular high risk of developing liver cirrhosis in people who are living with HIV (Calle-Serrano 2012, Fernandez-Montero 2014). In one cross-sectional study from Spain, 66% of people coinfected with HIV/HBV/HCV/HDV presented with liver cirrhosis compared to only 6% of people coinfected with only HBV/HCV/HIV (Castellares 2008) and this translated to higher rates of liver decompensation and death (Fernandez-Montero 2014). Similarly, HDV was associated with poorer survival in HIV positive people in Taiwan (Sheng 2007, Lee 2013) and in the Swiss HIV cohort study (Beguelin 2017). The Swiss study showed a prevalence of HDV of 15.4% and showed a 2.3 fold increased risk of overall death for those coinfected with HIV/HDV. Of note, the association of HDV with mortality and liver-related complications including HCC was independent from ongoing drug injection or HCV coinfection.
An easy-to-apply clinical score, the baseline-event anticipation (BEA) score, has been suggested to predict the risk of developing liver-related morbidity and mortality (Calle-Serrano 2014). Factors associated with a poor long-term outcome included age above 40, male sex, low platelet counts, high bilirubin and INR values and southeast Mediterranean origin. The score differentiated patients with a benign (BEA-A), intermediate (BEA-B) and severe (BEA-C) mid-term course of HDV infection and is available on www.hepatitis-delta.org. Anti-HDV IgM testing may also be useful as anti-IgM levels are associated with activity of liver disease (Mederacke 2012). The majority of people with HDV test positive for HDV-specific IgM antibodies but IgM negative patients did not develop clinical complications in a retrospective-prospective follow-up study from Germany (Wranke 2014). Thus, clinical parameters may be used to decide if a patient should be considered for antiviral therapy with PEG-IFN α. Currently, treatment may be deferred for some time in individuals with a BEA-A score or in patients who are anti-HDV IgM negative.
Diagnosis of HDV
We recommend that everyone who is HBsAg positive be tested for anti-HDV antibodies at least once (Figure 4). There is currently no evidence that direct testing for HDV RNA in the absence of anti-HDV is of any use. A positive result for anti-HDV does not necessarily indicate active HDV, as HDV RNA can become negative indicating recovery from HDV infection. Also, over the long-term, anti-HDV antibodies can be lost after HDV recovery. However, anti-HDV may persist for years even when the patient has experienced HBsAg seroconversion and anti-HDV remains detectable in most patients even after liver transplantation when HBsAg and HDV RNA are cleared (Mederacke 2012).
Active replicative HDV should be confirmed by the detection of HDV RNA. If HDV RNA is positive, subsequent evaluation of grading and staging of liver disease, surveillance for hepatocellular carcinoma and consideration of antiviral treatment is indicated. HDV RNA quantification is offered by some laboratories. However, so far there is no consistent evidence that HDV RNA levels are strongly correlated with histological markers of liver disease (Zachou 2010) even though high HDV RNA levels may be predictive of developing cirrhosis and HCC in the long term (Romeo 2014). Another recent study in HDV genotype 3 infection also showed an association between HDV RNA levels and serum levels of liver enzymes (Braga 2014). HDV RNA quantification is useful in particular if antiviral treatment is indicated. Stopping rules during antiviral treatment depending on the level of antiviral decline are currently being evaluated. A WHO standard for HDV has been released which allows comparison of performances of various PCR assays that have been published in recent years (Mederacke 2010, Niro 2011, Katsoulidou 2013, Bothelo-Souza 2014). Even commercial assays may show limited performance in detecting and quantifying HDV RNA (Brichler 2013). An international quality assessment study involving 28 laboratories globally revealed a very high heterogeneity of assay characteristics (Le Gal 2016). Less than half of the laboratories quantified all HDV RNA positive samples and reported quantitative values varied largely between the laboratories.
HDV genotyping is performed by some research labs and may help to identify patients with a higher or lower risk of developing end-stage liver disease (Su 2006). In high-income countries, almost all patients are infected with HDV genotype 1, thus genotyping may be considered mainly in immigrants or populations with mixed genotype prevalence.
 Figure 4. Diagnostic steps in HDV
Figure 4. Diagnostic steps in HDV
During the 1980s and 1990s, the diagnosis of active HDV was dependent on anti-HDV IgM testing. Anti-HDV IgM testing might still be useful in patients who test HDV RNA negative but have evidence of liver disease, which cannot be explained by other reasons. Due to the variability of the HDV genome and the lack of standardisation of HDV RNA assays, HDV RNA may test false negative or be under the detection limit of the assay in the case of fluctuating viral load. In these cases, HDV RNA testing should be repeated and anti-HDV IgM testing might be performed, if available. Anti-HDV IgM levels also correlate with disease activity (Wranke 2014) and may be predictive for response to IFN α-based antiviral therapy (Mederacke 2012).
As HDV occurs only in the context of HBV coinfection, a solid work-up of HBV infection including HBV DNA quantification and HBeAg/anti-HBe determination is warranted. Between 10% and 20% of HDV patients are HBeAg positive. Of note, HBV DNA is suppressed even in HBeAg positive hepatitis (Heidrich 2012) suggesting that the inhibitory effect of HDV on HBV is independent from the phase of HBV infection. The long-term clinical outcome of anti-HDV positive patients did not differ between HBeAg positive and HBeAg negative individuals in one study from Germany (Heidrich 2012). Most HDV patients in Europe are infected with HBV genotype D but infection with genotype A can also occur (Soriano 2011) which may have significant implications for treatment decisions, as HBV genotype A shows a better response to interferon α therapy – which however needs to be confirmed in the context of HDV coinfection. Similarly, testing for anti-HCV and anti-HIV is mandatory. Up to one third of anti-HDV positive patients can also test positive for anti-HCV (Heidrich 2009).
Quantitative HBsAg levels correlate with HDV RNA levels in HDV infection (Shih 2008). Higher HBsAg levels may also indicate more severe histological disease activity (Zachou 2010). Thus, a determination of quantitative HBsAg values also has some clinical relevance in patients with HDV. Monitoring of quantitative HBsAg levels should be performed in all patients undergoing antiviral therapies as long-term interferon therapy of HDV should be individualised until HBsAg is lost (Heller 2014, Guedj 2014).
Staging of liver disease is of particular importance in HDV as treatment options are limited and as the only possible therapy interferon α can lead to frequent and sometimes severe side effects. Various non-invasive serum markers have been developed to predict liver fibrosis and cirrhosis in HCV, HBV and NASH. However, scores such as APRI, FIB-4 or AST/ALT ratio have to be used with caution in HDV infection as they are of limited value in HDV (Lutterkort 2017, Takyar 2017). Novel score specifically developed for HDV have been proposed. One socre is based on serum cholinesterase, gamma glutamyl transferase, albumin and age and has been validated in European patients (Lutterkort 2017). Another score included gamma-glutamyl transpeptidase, platelet count, alanine aminotransferase, and liver stiffness measurement (Da 2019). Transient elastography has been shown to be useful in staging HDV patients with similar cut-off values to determine liver cirrhosis as in HCV infection (e.g. 14 kPa) (Da 2019b).
Treatment of HDV
Nucleoside and nucleotide analogues
Several nucleoside and nucleotide analogues (NA) used for the treatment of HBV infection have no direct antiviral effects against HDV as HDV uses host polymerases for replication. Nevertheless, NAs have been studied in patients with HBV-HDV coinfection with the idea that beneficial effects may arise due to indirect mechanisms (Table 2).
.
Famciclovir, used in the 1990s to treat HBV (Wedemeyer 1999), had no significant antiviral activity against HDV in a Turkish trial (Yurdaydin 2002). Similarly, lamivudine was ineffective in trials of HDV (Wolters 2000, Niro 2005a, Yurdaydin 2008, Lau 1999b). Ribavirin alone or in combination with interferon also did not lead to increased rates of HDV RNA clearance (Niro 2005a, Gunsar 2005, Garripoli 1994). None of the patients treated with adefovir monotherapy for 12 months became HDV RNA negative in the HIDIT-1 trial (Wedemeyer 2011). Similarly, short-term entecavir treatment did not show significant activity against HDV (Kabacam 2012b) However, a long-term observational study of HIV positive people receiving antiretroviral therapy (ART) followed individuals coinfected with HBV/HDV/HIV for a median of more than six years. Over this time, a decline of HDV RNA from 7 log10 to 5.8 log10 was observed and 3 out of 16 patients became HDV RNA negative (Sheldon 2008). Thus, very long treatment with HBV polymerase inhibitors may lead to beneficial effects in coinfected, possibly due to a reduction of HBsAg levels (Figure 5). These earlier findings were confirmed in a recent report from the same group describing HDV RNA negativation in 10/19 patients after a median use of tenofovir-DF (TDF) of 58 months (Soriano 2014). It is interesting to note that HDV RNA declines were not associated with HBsAg declines in this analysis. Importantly, HDV RNA negative patients also showed improvements in liver stiffness values, while this was not the case in subjects who remained HDV RNA positive. A small case series seemed to confirm the observation that ART could modify the clinical course of HDV infection in HIV positive patients (Onali 2015). In the SWISS HIV cohort, tenofovir-containing ART was associated with relevant HDV RNA declines in 29% of patients and 14% had undetectable HDV RNA after 5 years (Beguelin 2017b). Future long-term trials will need to confirm these data in triple-infected individuals and potential mechanism need to be studied. One hypothesis is that tenofovir may induce interferon lambda (Murata 2019) which has been shown to exert also direct antiviral effects against HDV (Giersch 2017). Considering the favorable safety profile, TDF may be considered for patients with HDV in the absence of alternative treatment options - e.g., for interferon-intolerant patients. Still there is currently no evidence that nucleoside or nucleotide analogue therapy is associated with a reduction of clinical complications of HDV as recently shown in a German single centre study (Wranke 2017).
Table 2. Treatment options in HDV| Nucleos(t)ide analogues | |
| Famciclovir ineffective | Yurdaydin 2002 |
| Lamivudine ineffective | Wolters 2000, Lau 1999, Niro 2005a, Niro 2008, Yurdaydin 2008 |
| Ribavirin ineffective | Niro 2006, Garripoli 1994, Gunsar 2005 |
| Adefovir ineffective (12 months) | Wedemeyer 2011 |
| Entecavir ineffective (12 months) | Kabacam 2012b |
| Tenofovir no evidence of short-term effect; long-term treatment associated with HBsAg and HDV RNA decline in some patients | Sheldon 2008, Soriano 2014, Beguelin 2017b |
| Interferon α | |
| Sustained biochemical responses in 0–36% of patients Few studies with virological endpoints Treatment >12 months may be required | Farci 1994, Di Marco 1996, Niro 2005b, Yurdaydin 2008 |
| Higher IFN doses were associated with better survival in small study cohort | Farci 2004 |
One promising and surprising alternative to the currently approved HBV polymerase inhibitors may have been clevudine. Clevudine, a nucleoside analogue no longer in development for the treatment of HBV, was shown to inhibit HDV in woodchucks (Casey 2005). However, a first pilot trial showed no significant HDV RNA declines in humans (Yakut 2010).
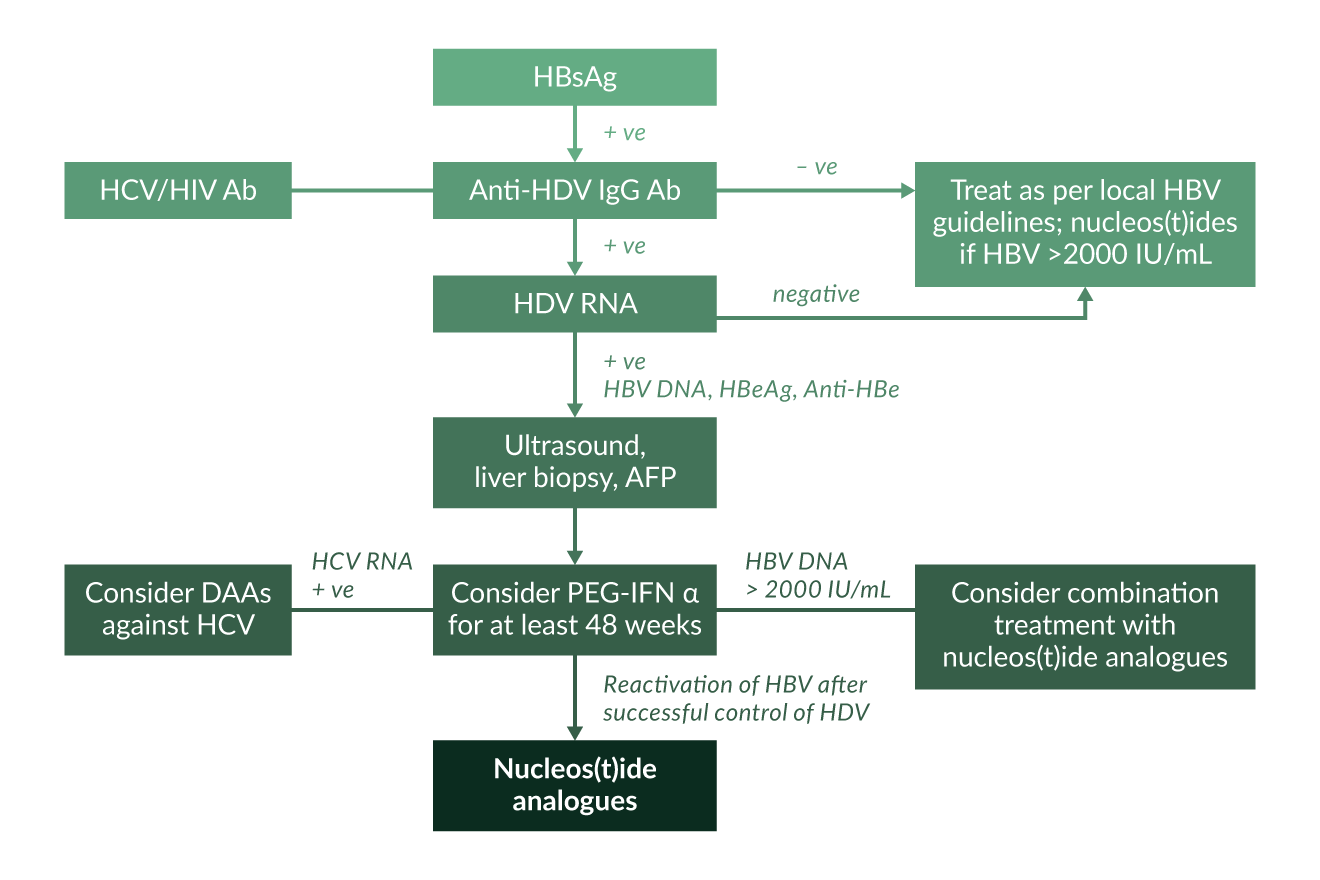 Figure 5. Treatment algorithm for HDV
Figure 5. Treatment algorithm for HDV
Recombinant interferon α
Interferon α has been used for the treatment of HDV since the mid-1980s (Rizzetto 1986). Since then, many trials have explored different durations and doses of interferon α in people with HDV. However, data are difficult to compare as endpoints are different in the trials and few studies have followed HDV RNA levels over time (Niro 2005b).
An Italian study reported a beneficial long-term outcome in hepatitis delta patients randomised to high-dose interferon α (Farci 1994, Farci 2004). These findings were confirmed by a retrospective single centre study showing that interferon-based antiviral therapy was an independent factor associated with a lower frequency of liver-related clinical complications (Wranke 2017, Yurdaydin 2018). Some studies have used extended doses of interferon treatment and it seems that two years of treatment is superior in terms of HDV RNA clearance (Niro 2005b). In a case report from the US NIH, 12 years of interferon treatment led finally to resolution of both HDV infection and HBsAg clearance (Lau 1999a). However, only minority of people can tolerate high doses of extended treatment interferon and treatment options are very limited for the majority (Manns 2006, Deterding 2019).
Pegylated interferon α
PEG-IFN α has been used in small trials to treat HDV, with post-treatment virological response rates of about 20% (Castelnau 2006, Niro 2006, Erhardt 2006) (Table 3).
Table 3. Pegylated interferon in hepatitis delta| Study | Course of therapy | Outcome* |
| Castelnau, Hepatology 2006 | 12 months of PEG-IFN α-2b (n=14) | FU24R in 6 patients (43%) |
| Niro, Hepatology 2006 | 72 weeks of PEG-IFN α-2b (n=38) – Monotherapy: n=16 – PEG-IFN + ribavirin during first 48 weeks: n=22 | FU24R in 8 patients (21%) Ribavirin had no additional effect |
| Erhardt, Liver Int 2006 | 48 weeks of PEG-IFN α-2b (n=12) | FU24R in 2 patients (17%) |
| Wedemeyer, NEJM 2011 | a) 48 weeks PEG-IFN α-2a + adefovir (n=31) or b) PEG-IFN α-2a + placebo (n=29) or c) adefovir (n=30) | FU24R Group a) 26% Group b) 31% Group c) 0% |
| Ormeci, Hepatogastroenterology 2011 | PEG-IFN α-2b 24 months (n=11) vs. 12 months (n=7) | No additional benefit of extended therapy |
| Karaca, Antivir Ther 2013 | 24 months PEG-IFN α-2a (n=32) | FU24R in 15 patients (47%) |
| Abbas, Antivir Ther 2014 | 48 weeks PEG-IFN α-2a (n=104) | FU24R in 24 patients (23%) |
| Wedemeyer, Lancet Infect Dis 2019 | 96 weeks PEG-IFN α-2a with or without tenofovir (n=120) | FU24R in 32 patients (27%) |
| Heller, AP&T 2014 | PEG-IFN α-2a for up to 5 years (dose up to 270 µg/week) | 4/12 patients HDV RNA negative, 3/12 HBsAg loss |
| Niro, AP&T 2016 | Treatment with PEG-IFNa. retrospective analysis of HBsAg kinetics | HBsAg and HDV RNA decline at month 6 predict long-term response |
Results of the Hep-Net International Delta hepatitis Intervention Trial (HIDIT-1) were published in 2011 (Wedemeyer 2011). 90 patients (42 in Germany, 39 in Turkey and 9 in Greece) with chronic HDV and compensated liver disease were randomised to receive either 180 µg PEG-IFN α-2a QW plus 10 mg adefovir dipivoxil QD (group A, n=31), 180 µg PEG-IFN α-2a QW plus placebo (group B, n=29) or 10 mg adefovir dipivoxil QD alone (group C, n=30) for 48 weeks. HBV DNA and HDV RNA were measured by real-time PCR. Ten patients did not complete 48 weeks of therapy because of disease progression (n=6) or interferon-associated side effects (n=4). Both PEG-IFN groups showed a significantly higher reduction in mean HDV RNA levels than the adefovir monotherapy group by week 48. HDV RNA was negative 24 weeks after the end of treatment in 28% of patients receiving PEG-IFN but in none of those treated with adefovir alone. While patients receiving PEG-IFN α-2a alone or adefovir monotherapy had similar mean HBsAg levels at week 0 and week 48, the PEG-IFN α-2a + adefovir combination group showed a 1.1 log10 IU/mL decline of HBsAg levels by week 48 (p<0.001) with 10/30 patients achieving a decline in HBsAg of more than 1 log10 IU/mL. These data are similar to a report from Greece of a significant decline in HbsAg levels in patients with HDV receiving long-term treatment with interferon α (Manesis 2007).
Overall the HIDIT-1 study showed that (i) PEG-IFN α-2a displays a significant antiviral efficacy against HDV in more than 40% of patients with about one fourth becoming HDV RNA negative after 48 weeks; (ii) adefovir dipivoxil has little efficacy in terms of HDV RNA reduction but may be considered for patients with significant HBV replication; (iii) combination therapy of PEG-IFN α-2a plus adefovir has no advantages for HBV DNA or HDV RNA reduction; (iv) a combination therapy of PEG-IFN + adefovir was superior to either monotherapy in reducing HBsAg levels in patients with HBV (Wedemeyer 2011). However, adefovir treatment was associated with a decline in glomerular filtration rates (Mederacke 2012) and thus PEG-IFN α + adefovir combination treatment cannot be recommended as first-line treatment for all patients with HDV. Treatment was safe and effective in patients with compensated liver cirrhosis (Kabacam 2012a), however treatment is not recommended in individuals with more advanced liver disease as liver decompensation may occur (Heidrich 2013). Overall, findings of the HIDIT-1 trial were largely in line with other subsequent studies of patients treated in Pakistan (Abbas 2014), Turkey (Ormeci 2011) or at the NIH in the United States (Heller 2014) (Table 3). PEG-IFN α induces HDV RNA suppression in about one quarter of patients, which may last for some time after the end of therapy. However, a long-term follow-up study of the HIDIT-1 trial showed that late HDV RNA relapses can occur in more than half of patients with a post-treatment week 24 response even though these were not associated with the development of clinical hepatic events (Heidrich 2014). Thus, regular long-term follow-up is required for all interferon-treated HDV patients irrespective of virologic response to therapy.
A small study involving 32 patients explored whether interferon lambda 3 (IFNL3, also known as interleukin 28B) polymorphisms are associated with response to interferon alfa-based therapies of HDV (Yilmaz 2014). Of note, IFNL3 did not affect treatment responses as sustained responses were 27%, 27% and 50% in patients with CC, CT and TT genotypes at rs12979860, respectively.
Additional trials have been performed to investigate the efficacy of PEG-IFN α-2a in combination with TDF for the treatment of HDV. First data of the HIDIT-2 trial were presented in 2013 showing that up to 47% of patients became HDV RNA negative after 96 weeks of PEG-IFN α-2a therapy irrespective of adding TDF or placebo (Wedemeyer 2019). In contrast to combination with adefovir, PEG-IFN α-2a plus TDF had no advantages in terms of HBsAg reduction after one year. However, relapses occurred after therapy and thus prolonged therapy may not necessarily prevent re-appearance of HDV and thus should not be considered in all patients unless pronounced HBsAg declines are observed – even though a smaller Turkish study reported rather high response rates of close to 50% after two years treatment (Karaca 2013). A long-term treatment study of up to five years in 13 patients at the NIH also observed a low virologic response rate despite prolonged therapy (Heller 2014). These findings suggest that therapy beyond one year is generally not beneficial although individual patients may benefit. If HBsAg kinetics can help to identify patients in whom longer treatment should be considered needs to be determined in future studies. Modelling data indicate that HBsAg-productive infected cells are the main source of HDV production (Guidj 2014) supporting the concept that treatment individualisation based on HBsAg levels during PEG-IFN α therapy is a reasonable approach. This is supported by a recent study which compared HDV patients who lost HBsAg during IFNa-based therapies compared to patients who were classified as partial responder (HBsAg positive, HDV RNA negative) or non-responder. A reduction of HBsAg at treatment month 6 was able to distinguish between the three groups (Niro 2016). Thus, determination of quantitative HBsAg is strongly recommended before and during PEG-IFN α therapy of HDV.
As PEG-IFN α therapy is of limited efficacy and as interferon-based therapies can cause significant side-effects, stopping rules would be helpful to avoid unnecessary PEG-IFN alfa exposure. Importantly, the HDV RNA level at week 24 of PEG-IFN α therapy can identify patients who will test HDV RNA negative during follow up after therapy (Keskin 2015). A decrease of HDV RNA of less than 1 log associated with no decline of HBsAg identified post-treatment non-responding patients with a positive predictive value of 83%. Another factor that is associated with response to PEG-IFN α therapy is the HBV-HDV dominance pattern before treatment. Interestingly, in the HIDIT-2 trial patients with higher HBV-DNA levels responded better to PEG-IFN α therapy compared to patients in whom HDV RNA levels were high and HBV DNA levels suppressed (Lutterkort 2018). Thus, the viral dominance pattern could be considered as a variable selecting patients for PEG-IFN α therapy .
New drugs against HDV in clinical development
Alternative treatment options for HDV are currently being explored in clinical trials (Wranke 2016; Deterding 2019). Treatment goals for new treatments against HDV have been suggested (Yurdaydin 2019). HDV RNA declines have been associated with improved clinical outcomes – even in the absence of complete negativation of HDV RNA. A preferred endpoint in clinical trials is a combination of HDV RNA decline with biochemical improvements of liver disease, e.g. normalization of ALT values (Yurdaydin 2019).
Prenylation inhibitors have been considered for quite some time (Bordier 2003). HDV replication depends on a prenylation step and prenylation inhibitors have already been developed for the treatment of malignancies. First proof-of-concept studies investigating the safety and efficacy of the prenylation inhibitor lonafarnib in patients with HDV have been initiated (www.clinicaltrials.gov) and indeed showed antiviral efficacy against HDV in patients (Koh 2015). Lonafarnib showed a dose-dependent reduction of HDV RNA levels of up to 2 log IU/mL after 28 days of therapy. Importantly, HDV RNA declines were associated with lonafarnib serum concentrations. While there was no evidence for viral resistance, higher doses of lonafarnib caused nausea and diarrhoea in most patients. Further trials on lonafarnib for HDV also exploring the potential of ritonavir boosting have been conducted suggesting that a better tolerated dose of lonafarnib may be possible (Yurdaydin 2018b). Furthermore, combination with PEG-IFN α-2a gave also promising results. A phase 3 trial is currently exploring both lonafarnib monotherapy as well as combination treatment with PEG-IFN α-2a in patients with HDV infection.
The HBV entry inhibitor bulevirtide (myrcludex-B) is also being developed for HDV. Bulevirtide is a lipopeptide derived from the preS1 domain of the HBV envelope and has been shown to hinder HDV infection in uPA/SCID mice transplanted with human hepatocytes (Lütgehetmann 2012). The molecular target of bulevirtide is the bile acid transporter sodium taurocholate cotransporting peptide (Ni 2013). The compound is also currently being tested in phase 1 and phase 2a trials in healthy volunteers and patients with HBV. 24 weeks of bulevirtide monotherapy was associated with an HDV RNA decline in the majority of patients in the first HDV study (Bogomolov 2016). Of note, patients receiving bulevirtide also showed a marked decline in ALT levels suggesting that blocking infection of cells can lead to a reduction in hepatitis activity. Additional trials exploring this compound in HDV alone or in combination with PEG-IFN α have already been completed. Overall, these studies confirmed a continuous and dose dependent linear HDV RNA decline during bulevirtide monotherapy associated with a marked improvement of biochemical disease activity (Deterding 2019). Of note, liver elastography values also improved in several patients. However, none of the patients experienced HBsAg losses when treated with bulevirtide alone. The safety profile of bulevirtide was excellent. By mode of action, bile acids increased during treatment, in particular in patients receiving the high dose of bulevirtide without causing any clinical symptoms. Combination treatment of bulevirtide with PEG-IFN α-2a led to synergistic effects against HDV and some patients also achieved HBsAg declines or even losses (Deterding 2019). A phase 3 program exploring the safety and efficacy of bulevirtide in patients with HDV infection is currently ongoing.
Finally, preliminary data have been presented for distinct nucleic acid polymers to treat patients with HDV (Bazinet 2017). Rep 2139-Ca is believed to block release of subviral HBsAg particles from hepatocytes. The compound was injected once weekly and induced a marked decline of HBsAg in some but not all patients with HDV treated in a centre in Moldova. Of note, all patients treated (n=12) showed an HDV RNA decline after 15 weeks of monotherapy when PEG-IFN α was added. Responses were maintained in seven patients one year after completing treatment. A transient ALT increase was observed in patients with low HBsAg levels after REP 2139 monotherapy when PEG-IFN α was introduced. Future studies will need to determine the efficacy and safety of REP 2139 in a larger group of patients with HDV infection.
Interferon lambda is also explored in patients with HDV infection, both as a monotherapy or in combination with lonafanib (Deterding 2019). In vitro and in humanized mice, an antiviral effect comparable to interferon alpha has been observerd (Giersch 2017). The potential advantage of interferon lambda is the lower frequency of systemic side effects as compared to interferon alpha.
Liver transplantation for HDV
Liver transplantation remains the ultimate treatment option for many people with HDV with end-stage liver disease. HDV patients have lower risk for reinfection after transplantation than patients with HBV monoinfection (Samuel 1993). If prophylaxis by passive immunisation with anti-HBs antibodies and administration of HBV polymerase is applied, HBV/HDV reinfection can be prevented in all individuals (Rosenau 2007) leading to an excellent long-term outcome after transplantation. HDV RNA levels rapidly decline during the first days after transplantation (Mederacke 2012) but HDVAg may persist in the transplanted liver for several years (Smedile 1998, Mederacke 2012). The possibility of reactivation of latent HDV infection by HBV superinfection has also been confirmed experimentally in a mouse model with transplanted human hepatocytes (Giersch 2014). Mice infected with HDV lacking HBV could be rescued by HBV superinfection after 2–6 weeks leading to a productive coinfection. Long-term prophylaxis to prevent HBV reinfection is therefore generally recommended in patients transplanted for HDV as reinfection may lead to HDV reactivation for which treatment options are very limited. Still, a recent report suggested that prophylaxis with nucleos(t)ides alone may be feasible as only 2 out of 34 patients had HBV/HDV recurrence when administration of HBV immunoglobulins was stopped after transplantation (Cholongitas 2016).
More information on HDV for physicians and patients can be found on the website of the Hepatitis Delta International Network: www.hepatitis-delta.org
References
Abbas Z. Hepatitis D in Pakistan. J Coll Physicians Surg Pak. 2012;22:547-8.
Abbas Z, Memon MS, Mithani H, Jafri W, Hamid S. Treatment of chronic hepatitis D patients with pegylated interferon: a real-world experience. Antivir Ther. 2014;19:463-8.
Abeywickrama-Samarakoon N, Cortay JC, Sureau C, et al.. Hepatitis Delta Virus histone mimicry drives the recruitment of chromatin remodelers for viral RNA replication.Nat Commun. 2020;11(1):419.
Alfaiate D, Lucifora J, Abeywickrama-Samarakoon N, et al. HDV RNA replication is associated with HBV repression and interferon-stimulated genes induction in super-infected hepatocytes. Antiviral Res. 2016;136:19-31.
Andernach IE, Leiss LV, Tarnagda ZS, et al. Characterization of hepatitis delta virus in sub-Saharan Africa. J Clin Microbiol. 2014;52:1629-36.
Aslan N, Yurdaydin C, Wiegand J, et al. Cytotoxic CD4 T cells in viral hepatitis. J Viral Hepat 2006;13:505-14.
Bazinet M, Pântea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol. 2017;2:877-889.
Benegiamo G, Vinciguerra M, Guarnieri V, Niro GA, Andriulli A, Pazienza V. Hepatitis delta virus induces specific DNA methylation processes in Huh-7 liver cancer cells. FEBS Lett. 2013;587:1424-8.
Béguelin C, Moradpour D, Sahli R, et al.Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol. 2017;66:297-303.
Béguelin C, Friolet N, Moradpour D, et al.. Impact of Tenofovir on Hepatitis Delta Virus Replication in the Swiss Human Immunodeficiency Virus Cohort Study. Clin Infect Dis. 2017;64:1275-1278.
Bogomolov P, Alexandrov A, Voronkova N,et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol. 2016;65:490-8.
Bordier BB, Ohkanda J, Liu P, et al. In vivo antiviral efficacy of prenylation inhibitors against hepatitis delta virus. J Clin Invest 2003;112:407-14.
Botelho-Souza LF, dos Santos Ade O, Borzacov LM, Honda ER, Villalobos-Salcedo JM, Vieira DS. Development of a reverse transcription quantitative real-time PCR-based system for rapid detection and quantitation of hepatitis delta virus in the western Amazon region of Brazil. J Virol Methods. 2014;197:19-24.
Braga WS, de Oliveira CM, de Araújo JR, et al. Chronic HDV/HBV co-infection: Predictors of disease stage - a case series of HDV-3 patients. J Hepatol. 2014;61:1205-11.
Brichler S, Le Gal F, Butt A, Chevret S, Gordien E. Commercial real-time reverse transcriptase PCR assays can underestimate or fail to quantify hepatitis delta virus viraemia. Clin Gastroenterol Hepatol. 2013;11:734-40.
Butí M, Homs M, Rodriguez-Frias F, et al. Clinical outcome of acute and chronic hepatitis delta over time: a long-term follow-up study. J Viral Hepat 2011; 18: 434-442.
Calle-Serrano B, Großhennig A, Homs M, et al. Development and Evaluation of a Baseline Event-Anticipation (BEA)-Score for Hepatitis Delta. Journal of Viral Hepatitis 2014 Nov;21(11):e154-63. doi: 10.1111/jvh.12251. Epub 2014 Mar 27.
Calle-Serrano B, Manns MP, Wedemeyer H. Hepatitis Delta in HIV-infected individuals. Seminars in Liver Disease 2012; 32: 120-9.
Casey J, Cote PJ, Toshkov IA, et al. Clevudine inhibits hepatitis delta virus viraemia: a pilot study of chronically infected woodchucks. Antimicrob Agents Chemother 2005;49:4396-9.
Casey JL, Niro GA, Engle RE, et al. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: the roles of HDV genotype III and HBV genotype F. J Infect Dis 1996;174:920-6.
Castellares C, Barreiro P, Martin-Carbonero L, et al. Liver cirrhosis in HIV-infected patients: prevalence, aetiology and clinical outcome. J Viral Hepat 2008;15:165-72.
Castelnau C, Le Gal F, Ripault MP, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology 2006;44:728-35.
Chen HY, Shen DT, Ji DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2018 pii: gutjnl-2018-316601. doi: 10.1136/gutjnl-2018-316601. [Epub ahead of print]as E, Goulis I, Antoniadis N, et al. . Nucleos(t)ide analog(s) prophylaxis after hepatitis B immunoglobulin withdrawal against hepatitis B and D recurrence after liver transplantation. Transpl Infect Dis. 2016;18:667-673.
Cross TJ, Rizzi P, Horner M, et al. The increasing prevalence of hepatitis delta virus (HDV) infection in South London. J Med Virol 2008;80:277-82.
Da BL, Surana P, Kleiner DE, et al. C. The Delta-4 fibrosis score (D4FS): A novel fibrosis score in chronic hepatitis D. Antiviral Res. 2019;174:104691.
Da BL, Surana P, Takyar V, et al. C. Vibration-controlled transient elastography for the detection of cirrhosis in chronic hepatitis D infection. J Viral Hepat. 2019 Nov 19. epub
Dienes HP, Purcell RH, Popper H, Ponzetto A. The significance of infections with two types of viral hepatitis demonstrated by histologic features in chimpanzees. J Hepatol 1990;10:77-84.
Degertekin H, Yalcin K, Yakut M, Yurdaydin C. Seropositivity for delta hepatitis in patients with chronic hepatitis B and liver cirrhosis in Turkey: a meta-analysis. Liver Int 2008;28:494-98.
Dény P. Hepatitis delta virus genetic variability: from genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol. 2006;307:151-71.
Deterding K, Pothakamuri SV, Schlaphoff V, et al. Clearance of chronic HCV infection during acute delta hepatitis. Infection 2009;37:159-62.
Deterding K, Wedemeyer H. Beyond Pegylated Interferon alpha: New treatments for hepatitis delta. AIDS Rev 2019; 21:126-34.
Dias J, Hengst J, Parrot T, et al. Chronic hepatitis delta virus infection leads to functional impairment and severe loss of MAITcells. J Hepatol. 2019;71:301-312.
El Bouzidi K, Elamin W, Kranzer K, et al. Hepatitis delta virus testing, epidemiology and management: a multicentre cross-sectional study of patients in London. J Clin Virol. 2015 ;66:33-7.
Erhardt A, Knuth R, Sagir A, Kirschberg O, Heintges T, Haussinger D. Socioepidemiological data on hepatitis delta in a German university clinic – increase in patients from Eastern Europe and the former Soviet Union. Z Gastroenterol 2003;41:523-6.
Erhardt A, Gerlich W, Starke C, et al. Treatment of chronic hepatitis delta with pegylated interferon-alpha2b. Liver Int 2006;26:805-10.
Erhardt A, Hoernke M, Heinzel-Pleines U, Sagir A, Göbel T, Häussinger D. Retrospective analysis of chronic hepatitis D in a Western German University Clinic over two decades: migratory pattern, prevalence and clinical outcome. Z Gastroenterol 2010; 48:813-7.
Farci P, Mandas A, Coiana A, et al. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med 1994;330:88-94.
Farci P, Roskams T, Chessa L, et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126:1740-9.
Farci P, Niro GA. Clinical features of hepatitis D. Semin Liver Dis. 2012; 32:228-36.
Fattovich G, Giustina G, Christensen E, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 2000;46:420-6.
Fernández-Montero JV, Vispo E, Barreiro P, et al. Hepatitis delta is a major determinant of liver decompensation events and death in HIV-infected patients. Clin Infect Dis. 2014; 58:1549-53.
Flodgren E, Bengtsson S, Knutsson M, et al. Recent high incidence of fulminant hepatitis in Samara, Russia: molecular analysis of prevailing hepatitis B and D virus strains. J Clin Microbiol 2000;38:3311-6.
Gaeta GB, Stroffolini T, Chiaramonte M, et al. Chronic hepatitis D: a vanishing Disease? An Italian multicenter study. Hepatology 2000;32(4 Pt 1):824-7.
Garripoli A, Di Marco V, Cozzolongo R, et al. Ribavirin treatment for chronic hepatitis D: a pilot study. Liver 1994;14:154-7.
Giersch K, Helbig M, Volz T, et al. Persistent hepatitis D virus mono-infection is efficiently converted by hepatitis B virus to a productive co-infection. J Hepatol 2014; 60: 538-44.
Giersch K, Allweiss L, Volz T, et al. Hepatitis delta coinfection in humanized mice leads to pronounced induction of innate responses in comparison to HBV monoinfection. J Hepatol 2015; 63: 346-53.
Giersch K, Homs M, Volz T, et al. M. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDVinfected humanized mice. Sci Rep. 2017; 7:3757.
Grabowski J, Wedemeyer H. Hepatitis delta: immunopathogenesis and clinical challenges. Dig Dis 2010;28:133-8.
Grabowski J, Yurdaydin C, Zachou K, et al. Hepatitis D virus-specific cytokine responses in patients with chronic hepatitis delta before and during interferon alfa-treatment. Liver Int 2011;31:1395-405.
Guedj J, Rotman Y, Cotler SJ, et al. Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. Hepatology. 2014;60:1902-10.
Gunsar F, Akarca US, Ersoz G, et al. Two-year interferon therapy with or without ribavirin in chronic delta hepatitis. Antivir Ther 2005;10:721-6.
Hadziyannis SJ. Review: hepatitis delta. J Gastroenterol Hepatol 1997;12:289-98.
Han M, Littlejohn M, Yuen L, et al. Molecular epidemiology of hepatitis delta virus in the Western Pacific region. J Clin Virol. 2014;61:34-9.
He W, Ren B, Mao F, et al. Hepatitis D Virus Infection of Mice Expressing Human Sodium Taurocholate Co-transporting Polypeptide. PLoS Pathog. 2015; 11:e1004840.
He W, Cao Z, Mao F, et al. Modification of Three Amino Acids in Sodium Taurocholate Cotransporting Polypeptide Renders Mice Susceptible to Infection with Hepatitis D Virus In Vivo. J Virol. 2016;90:8866-74.
Heidrich B, Deterding K, Tillmann HL, Raupach R, Manns MP, Wedemeyer H. Virological and clinical characteristics of delta hepatitis in Central Europe. J Viral Hepat 2009;16:883-94.
Heidrich B, C Serrano B, Idilman R, et al. HBeAg positive hepatitis delta: virological patterns and clinical long-term outcome HBeAg positive hepatitis delta. Liver Int 2012:32:1415-25.
Heidrich B, Manns MP, Wedemeyer H. Treatment Options for Hepatitis Delta Virus Infection. Curr Infect Dis Rep 2013;15:31-8.
Heidrich B, Yurdaydın C, Kabaçam G, et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology. 2014; 60:87-97.
Heller T, Rotman Y, Koh C, et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther. 2014; 40:93-104.
Hershow RC, Chomel BB, Graham DR, et al. Hepatitis D virus infection in Illinois state facilities for the developmentally disabled. Epidemiology and clinical manifestations. Ann Intern Med 1989;110:779-85.
Homs M, Rodriguez-Frias F, Gregori J, et al. Evidence of an Exponential Decay Pattern of the Hepatitis Delta Virus Evolution Rate and Fluctuations in Quasispecies Complexity in Long-Term Studies of Chronic Delta Infection. PLoS One. 2016;11(6):e0158557.
Huang YH, Tao MH, Hu CP, Syu WJ, Wu JC. Identification of novel HLA-A*0201-restricted CD8+ T-cell epitopes on hepatitis delta virus. J Gen Virol 2004;85(Pt 10):3089-3098.
Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011;378:73-85.
Hung CC, Wu SM, Lin PH, et al. Increasing incidence of recent hepatitis D virus infection in HIV-infected patients in an area hyperendemic for hepatitis B virus infection. Clin Infect Dis. 2014; 58:1625-33.
Ippolito AM, Niro GA, Fontana R, et al. Unawareness of HBV infection among inpatients in a Southern Italian hospital. J Viral Hepatitis 2011; 18:e206-211.
Kabaçam G, Dalekos GN, Cakaloğlu Y, et al. Pegylated interferon-based treatment in patients with advanced liver disease due to chronic delta hepatitis. Turk J Gastroenterol. 2012a; 23:560-8.
Kabacam G, Onder FO, Yakut M, et al. Entecavir treatment of chronic hepatitis D. Clin Infect Dis 2012b, 55: 645-650.
Karaca C, Soyer OM, Baran B, et al. Efficacy of pegylated interferon-α treatment for 24 months in chronic delta hepatitis and predictors of response. Antivir Ther. 2013;18:561-6.
Karataylı SC, Bozdayı M, Karataylı E, et al. Interleukin-28 gene polymorphisms may contribute to HBsAg persistence and the development of HBeAg negative chronic hepatitis B. Liver Int. 2015; 35:846-53.
Karimzadeh H, Kiraithe MM, Kosinska et al. Amino Acid Substitutions within HLA-B*27-Restricted T Cell Epitopes Prevent Recognition by Hepatitis Delta Virus-Specific CD8+ T Cells. J Virol. 2018;92 (13) epub.
Karimzadeh H, Kiraithe MM, Oberhardt V, et al. Mutations in Hepatitis D Virus Allow It to Escape Detection by CD8+ T Cells and Evolve at the Population Level. Gastroenterology. 2019;156:1820-1833.
Katsoulidou A, Manesis E, Rokka C, et al. Development and assessment of a novel real-time PCR assay for quantitation of hepatitis D virus RNA to study viral kinetics in chronic hepatitis D. J Viral Hepat. 2013; 20:256-62.
Kay A, Melo da Silva E, Pedreira H, et al. HBV/HDV co-infection in the Western Brazilian Amazonia: an intriguing mutation among HDV genotype 3 carriers. J Viral Hepat. 2014;21:921-4.
Kefalakes H, Koh C, Sidney J, et al. Hepatitis D Virus-Specific CD8+ T Cells Have a Memory-Like Phenotype Associated With Viral Immune Escape in Patients With Chronic Hepatitis D Virus Infection. Gastroenterology. 2019;156:1805-1819
Keskin O, Wedemeyer H, Tüzün A, et al. .Association between level of HDV RNA at week 24 of pegyalted interferon therapy and outcome. Clin Gastroenterol Hepatol 2015; 13: 2342-49.
Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis. 2015;15:1167-74.
Kushner T, Serper M, Kaplan DE. Delta hepatitis within the veterans affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol 2015; 63: 586-92.
au DT (a), Kleiner DE, Park Y, Di Bisceglie AM, Hoofnagle JH. Resolution of chronic delta hepatitis after 12 years of interferon alfa therapy. Gastroenterology 1999;117:1229-33.
Landahl J, Bockmann JH, Scheurich C, et al. Detection of a Broad Range of Low-Level Major Histocompatibility Complex Class II-Restricted, Hepatitis Delta Virus (HDV)-Specific T-Cell Responses Regardless of Clinical Status. J Infect Dis. 2019; 219:568-577
Lau DT (b), Doo E, Park Y, et al. Lamivudine for chronic delta hepatitis. Hepatology 1999;30:546-9.
Lee CY, Tsai HC, Lee SS, et al. Higher rate of hepatitis events in patients with human immunodeficiency virus, hepatitis B, and hepatitis D genotype II infection: A cohort study in a medical center in southernTaiwan. J Microbiol Immunol Infect 2015; 48: 20-7.
Le Gal F, Castelneau C , Gault E, et al. Hepatitis D virus infection-not a vanishing disease in Europe! Hepatology 2007;45:1332-3.
Le Gal F, Brichler S, Sahli R, Chevret S, Gordien E. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology. 2016;64:1483-1494.
Le Gal F, Brichler S, Drugan T, et al. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology. 2017;66:1826-1841
Liao B, Zhang F, Lin S, et al. Epidemiological, Clinical and Histological Characteristics of HBV/HDV Co-Infection: A Retrospective Cross-Sectional Study in Guangdong, China. PLoS One. 2014;9(12):e115888.
Lin HH, Lee SS, Yu ML, et al. Changing hepatitis D virus epidemiology in a hepatitis B virus endemic area with a national vaccination program. Hepatology. 2015; 61:1870-9.
Lunemann S, Malone DF, Hengst J, et al. Compromised Function of Natural Killer Cells in Acute and Chronic Viral Hepatitis. J Infect Dis. 2014; 209: 1362-73.
Lunemann S, Malone DF, Grabowski J, et al. Effects of HDV infection and pegylated interferon α treatment on the natural killer cell compartment in chronically infected individuals. Gut 2015; 64:469-82.
Lütgehetmann M, Mancke LV, Volz T, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 2012;55:685-94.
Lutterkort GL, Wranke A, Yurdaydin C, et al. Non-invasive fibrosis score for hepatitis delta. Liver Int. 2017;37:196-204.
Lutterkort GL, Wranke A, Hengst J, et al. Viral dominance patterns in chronic hepatitis delta determine early response to interferon alpha therapy. J Viral Hepat. 2018;25:1384-1394.
Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55:1350-59.
Manesis EK, Schina M, Le Gal F, et al. Quantitative analysis of hepatitis D virus RNA and hepatitis B surface antigen serum levels in chronic delta hepatitis improves treatment monitoring. Antivir Ther 2007;12:381-8.
Manesis EK, Vourli G, Dalekos G, et al. Prevalence and clinical course of hepatitis delta infection in Greece: a 13 year prospective study. J Hepatol 2013; 59:949-59.
Mederacke I, Bremer B, Heidrich B, et al. Establishment of a novel quantitative hepatitis D virus (HDV) RNA assay using the Cobas TaqMan platform to study HDV RNA kinetics. J Clin Microbiol 2010;48:2022-9.
Mederacke I, Yurdaydin C, Großhennig A, et al. Renal function during treatment with adefovir plus peginterferon alfa-2a vs either drug alone in hepatitis B/D co-infection. J Viral Hepat 2012;19:387-395.
Mederacke I, Filmann N, Yurdaydin C, et al. Rapid early HDV RNA decline in the peripheral blood but prolonged intrahepatic hepatitis delta antigen persistence after liver transplantation. J Hepatol 2012;56:115-22.
Mederacke I, Yurdaydin C, Dalekos GN, et al. Anti-HDV immunoglobulin M testing in hepatitis delta revisited: correlations with disease activity and response to pegylated interferon-α2a treatment. Antivir Ther 2012;17:305-12.
Mele A, Mariano A, Tosti ME, et al. Acute hepatitis delta virus infection in Italy: incidence and risk factors after the introduction of the universal anti-hepatitis B vaccination campaign. Clin Infect Dis 2007; 44:e17-24.
Miao Z, Zhang S, Ou X, Li S, et al.Q. Estimating the global prevalence, disease progression and clinical outcome of hepatitis delta virus infection. J Infect Dis. 2019 Nov 28.
Murata K, Tsukuda S, Suizu F, et al.. Immunomodulatory Mechanism of Acyclic Nucleoside Phosphates in Treatment of Hepatitis BVirus Infection. Hepatology. 2019 Sep 17. epub
Nakano T, Shapiro CN, Hadler SC, et al. Characterization of hepatitis D virus genotype III among Yucpa Indians in Venezuela. J Gen Virol 2001;82(Pt 9):2183-2189.
Negro F, Bergmann KF, Baroudy BM, et al. Chronic hepatitis D virus (HDV) infection in hepatitis B virus carrier chimpanzees experimentally superinfected with HDV. J Infect Dis 1988;158:151-9.
Ni Y, Lempp FA, Mehrle S, et al. Hepatitis B and D Viruses Exploit Sodium Taurocholate Co-transporting Polypeptide for Species-specific Entry into Hepatocytes. Gastroenterology. 2013 Dec 18.
Niro GA, Casey JL, Gravinese E, et al. Intrafamilial transmission of hepatitis delta virus: molecular evidence. J Hepatol 1999;30:564-9.
Niro GA (a), Ciancio A, Tillman HL, et al. Lamivudine therapy in chronic delta hepatitis: a multicentre randomized-controlled pilot study. Aliment Pharmacol Ther 2005;22:227-32.
Niro GA (b), Rosina F, Rizzetto M. Treatment of hepatitis D. J Viral Hepat 2005;12:2-9.
Niro GA, Ciancio A, Gaeta GB, et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006;44:713-20.
Niro GA, Smedile A, Ippolito AM, et al. Outcome of chronic delta hepatitis in Italy: a long-term cohort study. J Hepatol 2010;53:834-40.
Niro GA, Gioffreda D, Fontana R. Hepatitis delta virus infection: open issues. Dig Liver Dis 2011;43 Suppl 1:S19-24.
Niro GA, Smedile A, Fontana R, et al. HBsAg kinetics in chronic hepatitis D during interferon therapy: on-treatment prediction of response. Aliment Pharmacol Ther. 2016; 44:620-8.
Nisini R, Paroli M, Accapezzato D, et al. Human CD4+ T-cell response to hepatitis delta virus: identification of multiple epitopes and characterization of T-helper cytokine profiles. J Virol 1997;71:2241-51.
Onali S, Figorilli F, Balestrieri C, et al. Can antiretroviral therapy modify the clinical course of HDV infection in HIV positive patients? Antivir Ther. 2015; 20:671-9.
Ormeci N, Bolukbas F, Erden E, et al. Pegylated interferon alfa-2B for chronic delta hepatitis: 12 versus 24 months. Hepatogastroenterology 2011;58:1648-53.
Perez-Vargas J, Amirache F, Boson B, et al. Enveloped viruses distinct from HBV induce dissemination of hepatitis D virus in vivo. Nat Commun. 2019;1:2098
Radjef N, Gordien E, Ivaniushina V, et al. Molecular phylogenetic analyses indicate a wide and ancient radiation of African hepatitis delta virus, suggesting a deltavirus genus of at least seven major clades. J Virol 2004;78:2537-44.
Raimondo G, Brunetto MR, Pontisso P, et al. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology 2006;43:100-7.
Rizzetto M. The delta agent. Hepatology 1983;3:729-37.
Rizzetto M, Rosina F, Saracco G, et al. Treatment of chronic delta hepatitis with alpha-2 recombinant interferon. J Hepatol 1986;3 Suppl 2:S229-33.
Rizzetto M. Hepatitis D: thirty years after. J Hepatol 2009;50:1043-50.
Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629-38.
Romeo R, Foglieni B, Casazza G, Spreafico M, Colombo M, Prati D. High serum levels of HDV RNA are predictors of cirrhosis and liver cancer in patients with chronic hepatitis delta. PLoS One. 2014; 9:e92062.
Rosenau J, Kreutz T, Kujawa M, et al. HBsAg level at time of liver transplantation determines HBsAg decrease and anti-HBs increase and affects HBV DNA decrease during early immunoglobulin administration. J Hepatol 2007;46:635-44.
Samuel D, Muller R, Alexander G, et al. Liver transplantation in European patients with the hepatitis B surface antigen. N Engl J Med 1993;329:1842-7.
Schaper M, Rodriguez-Frias F, Jardi R, et al. Quantitative longitudinal evaluations of hepatitis delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol 2010;52:658-64.
Schirdewahn T, Grabowski J, Owusu Sekyere S, et al. The Third Signal Cytokine Interleukin 12 Rather Than Immune Checkpoint Inhibitors Contributes to the Functional Restoration of Hepatitis D Virus-Specific T Cells. J Infect Dis. 2017;215:139-149.
Servant-Delmas A, Le Gal F, Gallian P, Gordien E, Laperche S. Increasing prevalence of HDV/HBV infection over 15 years in France. J Clin Virol 2014 Feb;59(2):126-8. doi: 10.1016/j.jcv.2013.11.016. Epub 2013 Dec 7.
Sheldon J, Ramos B, Toro C, et al. Does treatment of hepatitis B virus (HBV) infection reduce hepatitis delta virus (HDV) replication in HIV-HBV-HDV-coinfected patients? Antivir Ther 2008;13:97-102.
Sheng WH, Hung CC, Kao JH, et al. Impact of hepatitis D virus infection on the long-term outcomes of patients with hepatitis B virus and HIV coinfection in the era of highly active antiretroviral therapy: a matched cohort study. Clin Infect Dis 2007;44:988-95.
Shih HH, Jeng KS, Syu WJ, et al. Hepatitis B surface antigen levels and sequences of natural hepatitis B virus variants influence the assembly and secretion of hepatitis d virus. J Virol. 2008;82:2250-64.
Shirvani-Dastgerdi E, Pourkarim MR, Herbers U, et al. Hepatitis delta virus facilitates the selection of hepatitis B virus mutants in vivo and functionally impacts on their replicative capacity in vitro. Clin Microbiol Infect. 2016;22 : 98.e1-6.
Smedile A, Casey JL, Cote PJ, et al. Hepatitis D viraemia following orthotopic liver transplantation involves a typical HDV virion with a hepatitis B surface antigen envelope. Hepatology 1998;27:1723-9.
Soriano V, Grint D, d’Arminio Monforte A, et al. Hepatitis delta in HIV-infected individuals in Europe. AIDS 2011;25:1987-92.
Soriano V, Vispo E, Sierra-Enguita R, et al. Efficacy of prolonged tenofovir therapy on hepatitis delta in HIV-infected patients. AIDS. 2014;28:2389-94.
Spaan M, Carey I, Bruce M, et al. Hepatitis delta genotype 5 is associated with favourable disease outcome and better response to treatment compared to genotype 1.J Hepatol. 2020; epub Jan 22
Su CW, uang YH, Huo TI, et al. Genotypes and viraemia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology 2006;130:1625-35.
Sureau C, Negro F. The hepatitis delta virus: Replication and pathogenesis. J Hepatol. 2016; 64(1 Suppl):S102-S116
Takyar V, Surana P, Kleiner DE, et al. Noninvasive markers for staging fibrosis in chronic delta hepatitis. Aliment Pharmacol Ther. 2017;45:127-138.
Taylor JM. Virology of hepatitis D virus. Sem Liver Dis 2012 ;32:195-200.
Tsatsralt-Od B, Takahashi M, Nishizawa T, Endo K, Inoue J, Okamoto H. High prevalence of dual or triple infection of hepatitis B, C, and delta viruses among patients with chronic liver disease in Mongolia. J Med Virol 2005;77:491-99.
Tsatsralt-Od B, Takahashi M, Endo K, et al. Infection with hepatitis A, B, C, and delta viruses among patients with acute hepatitis in Mongolia. J Med Virol 2006;78:542-50.
Troisi CL, Hollinger FB, Hoots WK, et al. A multicenter study of viral hepatitis in a United States hemophilic population. Blood 1993;81:412-18.
Veloso Alves Pereira I, Buchmann B, Sandmann L, et al. Primary biliary acids inhibit hepatitis D virus (HDV) entry into human hepatoma cells expressing the sodium-taurocholate cotransporting polypeptide (NTCP). PLoS One. 2015; 10:e0117152.
Wedemeyer H, Boker KH, Pethig K, et al. Famciclovir treatment of chronic hepatitis B in heart transplant recipients: a prospective trial. Transplantation 1999;68:1503-11.
Wedemeyer H, Heidrich B, Manns MP. Hepatitis D virus infection - Not a vanishing disease in Europe! Hepatology 2007;45:1331-1332.
Wedemeyer H, Manns MP. Epidemiology, pathogenesis and management of hepatitis D: update and challenges ahead. Nat Rev Gastroenterol Hepatol 2010;7:31-40.
Wedemeyer H. Re-emerging interest in hepatitis delta: new insights into the dynamic interplay between HBV and HDV. J Hepatol 2010;52:627-9.
Wedemeyer H, Yurdaydìn C, Dalekos GN, et al. Pegylated interferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011; 364:322-31.
Wedemeyer H, Yurdaydin C, Hardtke S, et al. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis. 2019 Mar;19(3):275-286.
Wedemeyer H, Negro F. Devil Hepatitis D: an orphan disease or largely underdiagnosed? Gut 2018; epub
Weisfuse IB, Hadler SC, Fields HA, et al. Delta hepatitis in homosexual men in the United States. Hepatology 1989;9:872-4.
Weller ML, Gardener MR, Bogus ZC, et al. Hepatitis Delta Virus Detected in Salivary Glands of Sjögren’s Syndrome Patients and Recapitulates a Sjögren’s Syndrome-Like Phenotype in Vivo.Pathog Immun. 2016;1:12-40.
Williams V, Brichler S, Radjef N, et al. Hepatitis delta virus proteins repress hepatitis B virus enhancers and activate the alpha/beta interferon-inducible MxA gene. J Gen Virol 2009;90:2759-67.
Wolters LM, van Nunen AB, Honkoop P, et al. Lamivudine-high dose interferon combination therapy for chronic hepatitis B patients co-infected with the hepatitis D virus. J Viral Hepat 2000;7:428-34.
Wranke A, Heidrich B, Ernst S, et al; HIDIT-2 Study Group. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One. 2014; 9:e101002.
Wranke A, Wedemeyer H. Antiviral therapy of hepatitis delta virus infection - progress and challenges towards cure. Curr Opin Virol. 2016;20:112-118.
Wranke A, Serrano BC, Heidrich B, et al. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology. 2017 Feb;65(2):414-425. doi: 10.1002/hep.28876. Epub 2016 Nov 30.
Yakut M, Seven G, Baran B, et al. Clevudine treatment of chronic delta hepatitis. J Hepatol 2010; 52 Suppl 1:473.
Yan H, Peng B, Liu Y, et al. Viral entry of hepatitis B and D viruses and bile salts transportation share common molecular determinants on sodium taurocholate cotransporting polypeptide. J Virol. 2014; 88:3273-84.
Yilmaz E, Baran B, Soyer OM, Onel M, et al. Effects of polymorphisms in interferon λ 3 (interleukin 28B) on sustained virologic response to therapy in patients with chronic hepatitis D virus infection. Clin Gastroenterol Hepatol. 2014; 12:1753-8.
Yurdaydin C, Bozkaya H, Gurel S, et al. Famciclovir treatment of chronic delta hepatitis. J Hepatol 2002;37:266-71.
Yurdaydin C (b), Bozkaya H, Onder FO, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat 2008;15:314-21.
Yurdaydin C, Keskin O, Kalkan Ç, Karakaya et al. Interferon Treatment Duration in Patients With Chronic Delta Hepatitis and its Effect on the Natural Course of the Disease.J Infect Dis. 2018; 217:1184-1192.
Yurdaydin C, Keskin O, Kalkan Ç, et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology. 2018;67:1224-1236.
Yurdaydin C, Abbas Z, Buti M, et al. Hepatitis Delta International Network (HDIN). Treating chronic hepatitis delta: The need for surrogate markers of treatment efficacy. J Hepatol. 2019;70:1008-1015.
Zachou K, Yurdaydin C, Drebber U, et al. Quantitative HBsAg and HDV-RNA levels in chronic delta hepatitis. Liver Int 2010;30:430-7.
